Article Text
Abstract
Background and objectives Percutaneous peripheral nerve stimulation (PNS) is an analgesic modality involving the insertion of a lead through an introducing needle followed by the delivery of electric current. This modality has been reported to treat chronic pain as well as postoperative pain following knee and foot surgery. However, it remains unknown if this analgesic technique may be used in ambulatory patients following upper extremity surgery. The purpose of this proof-of-concept study was to investigate various lead implantation locations and evaluate the feasibility of using percutaneous brachial plexus PNS to treat surgical pain following ambulatory rotator cuff repair in the immediate postoperative period.
Methods Preoperatively, an electrical lead (SPR Therapeutics, Cleveland, Ohio) was percutaneously implanted to target the suprascapular nerve or brachial plexus roots or trunks using ultrasound guidance. Postoperatively, subjects received 5 min of either stimulation or sham in a randomized, double-masked fashion followed by a 5 min crossover period, and then continuous stimulation until lead removal postoperative days 14–28.
Results Leads (n=2) implanted at the suprascapular notch did not appear to provide analgesia, and subsequent leads (n=14) were inserted through the middle scalene muscle and placed to target the brachial plexus. Three subjects withdrew prior to data collection. Within the recovery room, stimulation did not decrease pain scores during the first 40 min of the remaining subjects with brachial plexus leads, regardless of which treatment subjects were randomized to initially. Seven of these 11 subjects required a single-injection interscalene nerve block for rescue analgesia prior to discharge. However, subsequent average resting and dynamic pain scores postoperative days 1–14 had a median of 1 or less on the Numeric Rating Scale, and opioid requirements averaged less than 1 tablet daily with active stimulation. Two leads dislodged during use and four fractured on withdrawal, but no infections, nerve injuries, or adverse sequelae were reported.
Conclusions This proof-of-concept study demonstrates that ultrasound-guided percutaneous PNS of the brachial plexus is feasible for ambulatory shoulder surgery, and although analgesia immediately following surgery does not appear to be as potent as local anesthetic-based peripheral nerve blocks, the study suggests that this modality may provide analgesia and decrease opioid requirements in the days following rotator cuff repair. Therefore, it suggests that a subsequent, large, randomized clinical trial with an adequate control group is warranted to further investigate this therapy in the management of surgical pain in the immediate postoperative period. However, multiple technical issues remain to be resolved, such as the optimal lead location, insertion technique, and stimulating protocol, as well as preventing lead dislodgment and fracture.
Trial registration number NCT02898103.
- neuromodulation:peripheral nerve stimulation
- postoperative pain
- acute pain
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, an indication of whether changes were made, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0
Statistics from Altmetric.com
Introduction
Rotator cuff repair frequently results in pain that is difficult to control with current analgesic options, particularly when it is performed as an outpatient procedure.1 One potential analgesic technique used historically to relieve chronic pain is neuromodulation involving the delivery of electric current to a target nerve,2 3 suggesting that this modality may also provide analgesia in the acute pain setting. Since conventional systems for peripheral nerve stimulation (PNS) usually require invasive surgery to both implant and remove multiple electrodes in close proximity to the peripheral nerve,4 5 the intrusiveness and cost of applying neuromodulation have been a major barrier to use of this modality to treat acute pain syndromes.6
However, a percutaneous lead (figure 1A) and wearable stimulator (figure 1B) were recently cleared by the US Food and Drug Administration to treat both chronic and acute postoperative pain with a lead implantation period of up to 60 days,7 8 affording the possibility of providing a non-opioid analgesic that outlasts surgical procedure-related pain.6 This system has been reported in small series of patients used to target the femoral and sciatic nerves following surgical procedures of the knee and foot.9–12 However, to our knowledge, percutaneous PNS to provide postoperative analgesia has never been reported involving the nerves of the upper extremity.
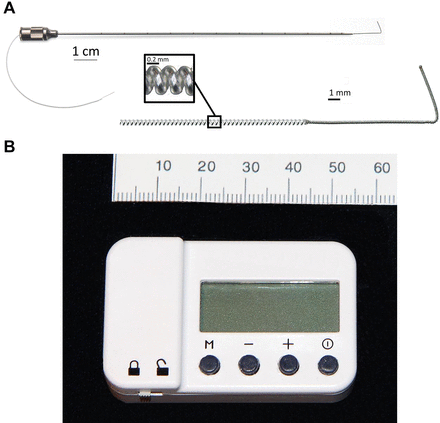
The percutaneous peripheral nerve stimulation equipment used for this study: a 12.5 cm, 20 G needle with a preloaded, helically coiled monopolar insulated electrical lead (panel A: MicroLead, SPR Therapeutics, Cleveland, Ohio), and a pulse generator or “stimulator” (panel B; SPR Therapeutics). Both illustrations were used with permission from BMI).
We therefore conducted a proof-of-concept study to evaluate the feasibility of providing percutaneous PNS of the suprascapular nerve and roots/trunks of the brachial plexus following ambulatory rotator cuff repair in the immediate postoperative period. A brief randomized, double-masked, sham-controlled, partial-crossover study was performed within the recovery room, followed by active open-label stimulation for all subjects for 14–28 days on an outpatient basis.
Methods
This study adhered to Good Clinical Practice quality standards and ethical guidelines defined by the Declaration of Helsinki. Study protocol approval as well as data and safety oversight were conducted by the University of California San Diego Institutional Review Board (IRB #151094; San Diego, California). Written, informed consent was obtained from all subjects participating in the trial. The trial was prospectively registered at ClinicalTrials.gov (principal investigator: BMI; date of registration: September 13, 2016) prior to initiation of enrollment. This study adhered to Good Clinical Practice quality standards and ethical guidelines defined by the Declaration of Helsinki. Study protocol approval as well as data and safety oversight were conducted by the University of California San Diego Institutional Review Board (IRB #151094; San Diego, California). Written, informed consent was obtained from all subjects participating in the trial.
Enrollment was offered to adults at least 18 years old scheduled for primary, unilateral, rotator cuff repair. Exclusion criteria included chronic opioid use (daily use within the 2 weeks prior to surgery and duration of use greater than 4 weeks); neuromuscular deficit within the operative extremity; anticipated MRI within the following 2 weeks; compromised immune system based on medical history or other conditions that increase the risk of infection; implanted spinal cord stimulator, cardiac pacemaker/defibrillator, deep brain stimulator, or other implantable neurostimulator; history of bleeding disorder; antiplatelet or anticoagulation therapies other than aspirin; allergy to local anesthetics, occlusive dressings, tape, or bandages; incarceration; or pregnancy.
Leads were implanted within 1 week prior to surgery. Subjects were positioned either seated (suprascapular) or supine (brachial plexus) and had the lead site prepared with chlorhexidine gluconate/isopropyl alcohol solution and sterile drapes. Given that one of the aims of this feasibility study was to investigate various lead implantation locations, the needle insertion points varied. Immediately prior to lead insertion, grip muscle strength was evaluated with an isometric force electromechanical dynamometer (Jamar Plus+ Hand Dynamometer, Sammons Preston, Bolingbrook, Illinois) to measure the force produced during a maximum voluntary isometric contraction during flexion of all fingers. The dynamometer was placed within the subject’s hand and the subject asked to take 2 s to come to a maximum effort flexing the fingers, maintain this effort for 5 s, and then relax.
Lead placement technique
A portable ultrasound (M-Turbo, SonoSite, Bothell, Washington) and linear array transducer (HFL38x, SonoSite) within a sterile sleeve were used for lead implantation. The suprascapular nerve at the suprascapular notch or brachial plexus at the level of the roots or trunks was imaged in a transverse cross-sectional (short axis) view. For suprascapular leads, the ultrasound transducer was parallel to the spine of the scapula and a local anesthetic skin weal raised medial to the transducer. For brachial plexus leads, the transducer was approximately within the parasagittal plane and a local anesthetic skin weal was raised posterolateral to the ultrasound transducer. For the first 14 subjects, a 12.5 cm, 20 G needle with a preloaded, helically coiled, insulated lead (MicroLead, SPR Therapeutics, Cleveland, Ohio) was inserted through the skin weal (figure 1A) and advanced to within 2 cm of the target nerve. For the last two subjects, a similar preloaded stimulating lead was used as part of a multicomponent implantation system (OnePass, SPR Therapeutics; figure 2A). The lead (MicroLead) or stimulating probe (OnePass system) was subsequently attached to an external pulse generator or “stimulator” (SPRINT, SPR Therapeutics) with a surface return electrode (figure 1B) placed on the ipsilateral limb.
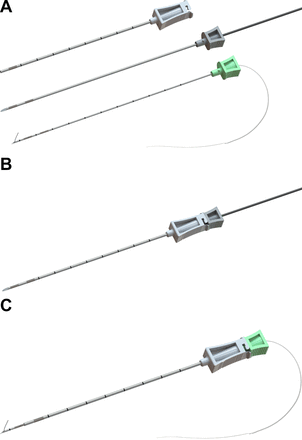
A multicomponent lead implantation system with (from top to bottom) an introducing sleeve, stimulating probe, and needle with preloaded lead (panel A: OnePass, SPR Therapeutics, Cleveland, Ohio; illustration used with permission from BMI). The stimulating probe is inserted and locked within the introducing sleeve (panel B) and positioned to produce the desired patient response; the probe is removed and replaced with the preloaded needle and lead (panel C), and the sleeve–needle withdrawn in tandem deploying the lead.
Stimulation was delivered with a square waveform at 100 Hz, and amplitude (range: 0.2–20 mA) and pulse duration (range: 15–200 μs) were adjusted until the subject reported sensory changes in the ipsilateral limb or until muscle contractions occurred.8 The optimal sensory changes targeted the ipsilateral shoulder, and if changes occurred proximal to the shoulder or muscle contractions occurred the current was decreased to the minimum settings, the stimulator was switched off, and the needle/introducer was advanced.
This process was repeated until sensory changes (often described as a “pleasant massage”) were reported in the shoulder, or the needle tip was within 0.5 cm of the target nerve (whichever came first). If the latter occurred with the MicroLead system, an additional pass with a new lead at a different level or slightly different trajectory was attempted until the optimal sensory changes with stimulation were achieved. The original preloaded lead has a 1.5 cm anchor at its tip and is deployed by withdrawing the needle over the lead. However, the OnePass system permitted withdrawal of the stimulating probe–sleeve combination without (figure 2B) deployment of the lead and could therefore be repositioned until the desired placement was achieved. At that time the stimulating probe was withdrawn leaving the sleeve in place, and the preloaded introducing needle was inserted through the probe and locked into the sleeve (figure 2C). The introducing needle–sleeve combination was then withdrawn, deploying the lead.
The lead was again connected to the stimulator to ensure lead dislodgment did not occur during deployment (if so, a new lead was implanted). Wound closure adhesive (2-Octyl 2-cyanoacrylate) was applied to the exit point, a connector block attached to the lead approximately 2 cm from the skin entry point, the excess lead removed with a sterile scissors, and the lead entry site covered with a sterile dressing.
The lead was again connected to the stimulator and settings were recorded. During stimulation, the maximum voluntary isometric contraction during finger contraction was again assessed using the same technique described for the prestimulation measurement. The stimulator was removed, and the subject returned home with the only limitations being a prohibition on submerging the lead entry site in water and strenuous exercise. Throughout the study, subjects were asked to use the Numeric Rating Scale (NRS, 0–10, 0=no pain, 10=worst imaginable pain) to rate their level of pain.
Day of surgery
Prior to surgery, the lead was again attached to a stimulator and the current increased with the revised settings recorded. The stimulator allowed a minimum, intermediate, and maximum intensity to be set by the healthcare provider which was subsequently controlled by subjects. The stimulator was removed, and the lead connecting wire covered with gauze and an occlusive dressing for the surgery. For surgical anesthesia, subjects received a general anesthetic with inhaled volatile anesthetic in nitrous oxide and oxygen. Intravenous fentanyl, hydromorphone, and/or morphine were administered intraoperatively, as needed.
Randomization
Within the recovery room, baseline measurements were recorded, including pain score at the surgical site using the NRS, and sensory deficits over the deltoid evaluated (binary endpoint measured with an alcohol pad and von Frey filament, compared with the contralateral limb, with any decrease considered a positive finding). Subjects were randomized to one of two groups using computer-generated lists and opaque, sealed envelopes: an initial 5 min of either electrical stimulation or sham, followed by 5 additional minutes of the opposite treatment. Two separate stimulators were programmed with the intermediate preoperative settings, one set to deliver active stimulation and the other set to sham (the sham mode is available for this stimulator model and is identical in appearance to the setting which delivers current). The investigator recording the outcome measures and remaining masked to the treatment group was provided the initial “Stimulator A” by an assistant, attached it to the lead, and initiated the stimulator. All investigators, clinical healthcare providers, and the subjects were masked to the treatment group, except for the single assistant who opened the sealed envelope. The outcome measures were recorded every minute for 5 min, at which time the alternative “Stimulator B” was attached to the lead and initiated. The outcome measures were again recorded every minute for 5 min, after which a “Stimulator C” programmed to deliver the actual current for all subjects was initiated and endpoints measured after 5 and 30 min.
Beginning 30 min following Stimulator C initiation, subjects could receive intravenous fentanyl or hydromorphone prior to discharge and/or receive a single-injection interscalene nerve block (ropivacaine 0.5%, 20 mL, with epinephrine).13 Subjects and their caretakers were provided verbal and written instructions on stimulator/lead care and management. The contact information for an investigator available at all times during the treatment period was provided. Subjects were discharged home with a prescription for oxycodone 5 mg tablets, replacement lead dressings, enough stimulator batteries for the duration of treatment, and their lead in situ. To increase analgesia, subjects were instructed to first increase the stimulation level on their pulse generators and use oral opioids as a last resort.
Subjects were contacted by telephone daily for data collection on postoperative days (POD) 1–14, 30, and 90. Information included pain level at the surgical site, opioid consumption, perceived sensory deficits (cold and light touch) anywhere in the operative extremity, and perceived muscle strength decrease in the ipsilateral extremity. Subjects returned to the orthopedic clinic for lead withdrawal, which entailed an investigator removing the occlusive dressing and continuous, gentle traction on the lead, similar to a perineural catheter extraction.
Statistical analysis
This was a proof-of-concept study to demonstrate feasibility and generate data to help design and power a subsequent clinical trial. Therefore, a convenience sample of 16 subjects were enrolled and statistics were not applied to the data due to the small sample size. The anthropomorphic and preoperative lead/stimulator characteristics are presented (table 1) as both mean (SD) and median, interquartile, and 10th–90th percentiles.
Anthropomorphic and preoperative lead/stimulator characteristics (n=16)
Results
Sixteen subjects enrolled, and 15 had a lead implanted successfully without sedation and reporting minimal discomfort (tables 1 and 2). One subject with a pre-existing anxiety disorder experienced what appeared to be a panic attack and elected to withdraw from study participation. The surgical approach for all subjects was arthroscopic and not open. Leads (n=2) implanted at the suprascapular notch did not appear to provide any analgesia at any time during the first two postoperative weeks, and subsequent leads (n=14) were inserted through the middle scalene muscle and placed to target the brachial plexus: five posterior to the superior brachial plexus trunk, six adjacent to the C5 nerve root, and three posterior to the distal middle trunk. Finger flexion maximum voluntary isometric contraction (grip strength) remained essentially unchanged during stimulation compared with baseline values (table 1). For two subjects, the rotator cuff repair procedure was canceled after viewing the joint under anesthesia, and so both withdrew from the study anticipating a lack of postoperative pain.
Stimulation parameters
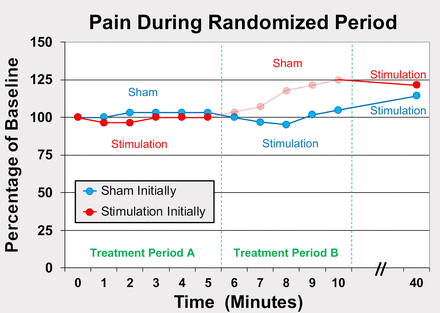
For the remaining 11 subjects with leads targeting the brachial plexus, stimulation did not decrease pain scores to any appreciable degree during the first 40 min within the recovery room, regardless of which treatment subjects were randomized to initially (figure 3). No sensory deficits (light touch or cold) or motor block was detected in any subject at any time point within the first 40 min following baseline. Following this time point, seven subjects (64%) requested supplemental opioids (median [range] intravenous morphine equivalent of 0.5 [0–3.5] mg), and seven subjects (64%) received a single-injection interscalene nerve block for rescue analgesia prior to discharge (only two of the eleven received neither opioid nor a nerve block).
Effects of percutaneous peripheral nerve stimulation of the brachial plexus on surgical pain within the recovery room immediately following rotator cuff repair. Subjects were randomized to receive 5 min of either electric current (“stimulation”; n=5) or sham (n=6) in a double-masked fashion (t reatment p eriod A) followed by a 5 min crossover period (treatment period B). Stimulation was subsequently delivered to all subjects (n=11) for 30 additional minutes. Data presented as mean at each time point, with the original pain scores measured using the Numeric Rating Scale. Given the relatively small sample size, statistics were not applied to the data. The group that received stimulation during the initial treatment has data shown in ghost during the subsequent period because peripheral nerve stimulation has a “carryover” effect and these data points are therefore difficult to interpret.
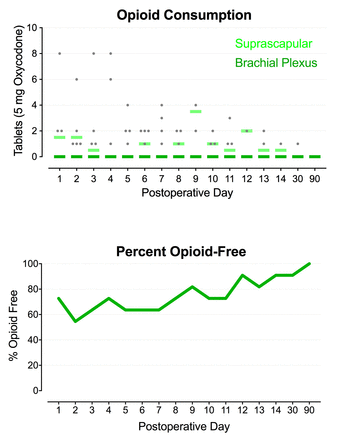
For subjects with leads targeting the brachial plexus, subsequent average resting and dynamic pain scores POD 1–14 had a median of 1 or less on the NRS (figure 4A); the median dynamic pain score was 3 or less (figure 4B); and opioid requirements averaged less than 1 tablet of oxycodone, 5 mg, daily with active stimulation (figure 5). No sensory deficits (light touch or cold) or motor block was detected by any subject at any time point during the follow-up period, with the exception of the duration of any administered interscalene nerve block. Leads were removed on POD 14–22 with two exceptions: one subject withdrew from the study the morning of POD 1 and another had the lead removed on POD 10 following a lead fracture.
Pain at rest and with movement during percutaneous peripheral nerve stimulation of the brachial plexus (n=11) or suprascapular nerve (n=2) following rotator cuff repair. Each circle represents one brachial plexus subject, and the median for each group at each time point is denoted with a horizontal line.
Opioid consumption and percent opioid-free during percutaneous peripheral nerve stimulation of the brachial plexus (n=11) or suprascapular nerve (n=2) following rotator cuff repair. Each circle represents one brachial plexus subject, and the median for each group at each time point is denoted with a horizontal line.
Adverse events and protocol deviations
The third subject (C) had his case canceled following lead implantation for reasons unrelated to himself, and the lead was removed and then subsequently replaced when his surgery was rescheduled. As described previously, the fourth subject (D) withdrew after experiencing what appeared to be a panic attack, and the fifth and sixth subjects (E and F) withdrew when their rotator cuff repair procedures were canceled intraoperatively. After 23 days, the lead of the ninth subject (I) partially unwound from its helical coil, the surgical adhesive was found dislodged 3 cm, and the lead stopped functioning, suggesting an accidental dislodgment. The tenth subject (J) experienced muscle contractions (unclear which muscles specifically) following resolution of her interscalene block and elected lead removal on POD 2 (with a significant subsequent increase in pain from a resting average of 1 to 6). The eleventh subject (K) accidentally pulled out his lead while removing his shirt on POD 8. The twelfth subject (L) purposefully removed his own lead at home without contacting a healthcare provider on POD 4 against instructions for use, and subsequently reported the 1.5 cm anchor fractured (he did not keep it for inspection by an investigator). Three other leads (E, G, and H) were found to be fractured following removal by a healthcare provider in clinic. All lead remnants were left in situ. No infections, nerve injuries, or adverse sequelae resulting from the lead fracture remnant were identified during the final two data collection phone calls on POD 30 or 90.
Discussion
This proof-of-concept study demonstrates that ultrasound-guided percutaneous PNS of the brachial plexus is feasible for ambulatory shoulder surgery. To our knowledge, it is the first report (1) of using percutaneous PNS to treat postoperative pain following surgical procedures of the shoulder; (2) of implanting a percutaneous lead in the region of the suprascapular nerve, as well as brachial plexus roots and trunks; and (3) providing data demonstrating a lack of PNS-induced sensory deficits or grip/muscle weakness of the upper extremity, exhibiting a mean (range) grip strength change from baseline of −0.7% (−0.9% to 2.5%).,
This lack of sensory and motor block is a potentially significant benefit compared with local anesthetic-based continuous peripheral nerve blocks, which can reduce the ability to participate in physical therapy and increase the risk of respiratory compromise. Furthermore, leads are implanted approximately 1–2 cm from the nerve compared with perineural catheters that are frequently inserted within the same fascial plane as the target nerve,9 10 theoretically decreasing the risk of needle-to-nerve contact and possible neurologic injury. Helically coiled electrical leads have a dramatically lower risk of infection than perineural catheters—fewer than 1 per 32,000 indwelling days14 15—and available pulse generators (“stimulators”) are now so small that they may be simply adhered to the patient’s skin with no infusion pump or large local anesthetic reservoir to carry. Combined with a historically lower dislodgment rate than perineural catheters, helically coiled leads are often used to provide PNS for multiple months and even years compared with the far more limited duration of continuous peripheral nerve blocks,16 which are typically used for only a few days.17 Benefits over opioids may include a lack of systemic side effects such as respiratory depression, nausea, and cognitive dysfunction, as well as potential for diversion, addiction, and abuse.18
Lead implantation location
Since percutaneous PNS has not previously been reported for shoulder surgery, one primary goal of the present study was to determine which anatomic locations were amenable to lead implantation. A recent abstract describes a case of percutaneous PNS targeting the suprascapular nerve to provide analgesia for the management of malignant neuropathic pain.19 Although we successfully implanted two leads targeting the suprascapular nerve at the suprascapular notch with concurrent sensory change in the shoulder joint, neither appeared to provide any appreciable analgesia at any postoperative time point. Whether this was due to an inadequate implantation technique or other reason remains unknown.
We implanted the subsequent five leads through the middle scalene with the tips approximately 1 cm posterior to the superior brachial plexus trunk using a similar technique to a posterior interscalene perineural catheter approach.20 These, at times, resulted in some cutaneous discomfort, suggesting a superficial location. We therefore implanted the subsequent six leads to target the C5 root of the brachial plexus, which was usually deeper as the root passed medially through the foramina. Unfortunately, this location frequently resulted in muscular contractions, most likely due to the multiple nerves originating from the brachial plexus in this region: dorsal scapular, long thoracic, supraclavicular, phrenic, as well as nerves to the longus colli and scalene muscles. Consequently, we implanted the remaining three leads posterior to the distal middle trunk in an attempt to avoid stimulating cutaneous fibers and inducing muscle contractions by overstimulating the suprascapular nerve and the nerve to the subclavius muscle, the latter two originating from the superior trunk. Determining the optimal lead location will require additional prospective investigation.
No lead location resulted in an appreciable downward trend in pain scores within 40 min of initiating stimulation within the recovery room (figure 3). It is tempting to conclude that PNS provides no analgesia during this period, except that sciatic and femoral PNS following hallux valgus and anterior cruciate ligament procedures demonstrated downward trends in pain scores of previous preliminary pilot studies.12 21 Although no conclusions may be drawn with the current limited data set, we speculate that in our subjects the perception and reporting of pain increased as sedation subsided following general anesthesia and the effects of intraoperative opioids diminished, and that pain scores may have been even higher without PNS. In other words, the increasing trend in pain scores within the first 40 min may not be due to complete lack of PNS-induced analgesia, but rather that the potency of pain control was inadequate for the degree of pain following rotator cuff repair (a majority of subjects required supplemental analgesics such as a single-injection interscalene nerve block within the recovery room).
For patients with persistent pain multiple weeks or months following knee arthroplasty in a previously-published series, analgesia was perceived within seconds of introducing electrical current via a femoral lead.9 10 However, additional experience with percutaneous PNS to treat acute pain suggests that maximum analgesia requires at least an hour of stimulation within the immediate postoperative period.12 21 22 Indeed, of the seven subjects who received a single-injection interscalene nerve block with ropivacaine following the initial 40 min of stimulation and prior to discharge, the mean (SD) pain scores and opioid tablet consumption recorded the following morning was 1.6 (1.3) and 1.7 (2.9), respectively, with all responding that their analgesia was adequate.
Lead design
The mean (SD) number of leads used per subject was 2.0 (2.1). Because these were the first leads ever used for shoulder surgery, we often attempted additional implantations in an effort to improve the location of induced sensory changes to/toward the shoulder, and many repeated implantations ultimately proved unnecessary. One of the limitations of the original lead design (figure 1A) is that the needle could not be withdrawn without deploying the lead. Therefore, instead of withdrawing and repositioning the needle/lead combination if a first attempt passed the target nerve without the desired response, an entirely new lead had to be implanted at a different level. For our final two subjects, a newer implantation system became available allowing probe withdrawal to optimize positioning prior to deployment of the lead itself, limiting the number of required leads to one unit per subject (figure 2).
Four leads fractured during withdrawal. Previous investigations involving the same helically coiled lead used in the current study had an approximate 9% average incidence of fracture during removal.8–10 12 21–32 It is notable that in three postoperative investigations including the present study, of 33 duplicate leads implanted and removed prior to use, not one fractured (0%); while, in contrast, of 31 leads inserted into the same subjects but used following surgery, 7 (23%) subsequently fractured.12 21 Combined with preliminary evidence that sciatic leads inserted at the popliteal fossa fracture at a far higher rate than sciatic leads inserted in the subgluteal region, we speculate that lead fracture is most likely related to applied tension due to repeated flexion and extension of the surrounding musculature. All previous fractured remnants have been left in situ with no negative sequelae reported in up to a 1-year period of assessment. Importantly, MRI may be performed safely in patients with retained lead fragments at 1.5 Tesla.33 Finally, most previously reported fractures occurred at or near the tip of the lead, leaving a relatively short remnant of less than 1.6 cm.33
Limitations
Prior experience with percutaneous PNS in postoperative subjects 8–97 days following knee arthroplasty suggested that analgesia onset and peak were nearly instantaneous following the introduction of electrical current.9 10 We therefore designed the current randomized, sham-controlled, crossover portion of this study with only 5 min treatment periods so that subjects randomized to sham initially would have a minimal duration without supplemental analgesia. However, results from investigations published in the interim suggest that for acute pain in the immediate postoperative period, maximum PNS-induced analgesia requires far longer than 5 min, possibly longer than 1 hour.12 21 Therefore, although the present study involved a 10 min randomized, crossover portion resulting in a control group, little can be determined from these data and a subsequent trial is required to produce an adequate treatment period.
In contrast, we were aware of a “carryover” effect following PNS so that subjects continue to receive a variable duration and degree of analgesia following electrical current discontinuation, possibly due to sustained modification of supraspinal pain processing.34 We knew that this carryover effect would make the data of the 5 min sham period for the group which initially received active current difficult or impossible to interpret. However, to keep the double-masked study design, we had no choice but to collect the measurements from this 5 min period. We therefore included the collected data but presented these in ghost to indicate the uncertainty of their interpretation (figure 3). Lastly, the optimal stimulation parameters remain unknown and the current results reflect the chosen parameters which were based on previous experience (table 2).9–12 However, other parameters might reduce the incidence of muscle contraction while improving analgesia, and further research is warranted in this regard.
Conclusions
This proof-of-concept study demonstrates that ultrasound-guided percutaneous PNS of the brachial plexus is feasible for ambulatory shoulder surgery, and although analgesia immediately following surgery does not appear to be as potent as local anesthetic-based peripheral nerve blocks, the study suggests that this modality may provide analgesia and decrease opioid requirements in the days following rotator cuff repair. The results of this pilot study indicate that a subsequent, large, randomized clinical trial with an adequate control group is warranted to further investigate this therapy in the management of surgical pain in the immediate postoperative period (initiated: NCT03481725). However, multiple technical issues remain to be resolved, such as the optimal lead location, insertion technique, and stimulating protocol, as well as preventing lead dislodgment and fracture.
Acknowledgments
The authors appreciate the invaluable assistance of Jacqueline Upton, MS (Department of Orthopedics, University of California San Diego, La Jolla, California); Madelyn Bernard, RN (Hillcrest Hospital, San Diego, California); and Baharin Abdullah (Clinical Translational Research Center, University of California San Diego, La Jolla, California).
References
Footnotes
Funding Funding for this project was provided by the University of California Academic Senate (San Diego, California) and the University of California San Diego Department of Anesthesiology (San Diego, California). SPR Therapeutics (Cleveland, Ohio) also provided the stimulators and leads used in this investigation. This company was given the opportunity to review the protocol and initial manuscript (minor revisions were suggested for each), but the investigators retained full control of the investigation, including study design, protocol implementation, data collection, analysis, and interpretation, and manuscript preparation.
Disclaimer The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the funding entities. None of the authors has a personal financial interest in this research.
Competing interests The University of California San Diego has received funding from SPR Therapeutics for other research studies of BMI, JJF, RAG, ETS, WBA, BK, JFS, MWS, PJ, DCC, and CMR.
Patient consent for publication Not required.
Ethics approval The study was approved by the UCSD Human Research Protections Program. This study adhered to Good Clinical Practice quality standards and ethical guidelines defined by the Declaration of Helsinki. Study protocol approval as well as data and safety oversight were conducted by the University of California San Diego Institutional Review Board (IRB #151094; San Diego, California). Written, informed consent was obtained from all subjects participating in the trial.
Provenance and peer review Not commissioned; externally peer reviewed.