Article Text
Abstract
We conducted a search of the literature to identify case reports of neuraxial and peripheral nervous system misconnection events leading to wrong-route medication errors. This narrative review covers a 20-year period (1999–2019; English-language publications and abstracts) and included the published medical literature (PubMed and Embase) and public access documents. Seventy-two documents representing 133 case studies and 42 unique drugs were determined relevant. The most commonly reported event involved administering an epidural medication by an intravenous line (29.2% of events); a similar proportion of events (27.7%) involved administering an intravenous medication by an epidural line. Medication intended for intravenous administration, but delivered intrathecally, accounted for 25.4% of events. In the most serious cases, outcomes were directly related to the toxicity of the drug that was unintentionally administered. Patient deaths were reported due to the erroneous administration of chemotherapies (n=16), muscle relaxants (n=4), local anesthetics (n=4), opioids (n=1), and antifibrinolytics (n=1). Severe outcomes, including paraplegia, paraparesis, spinal cord injury, and seizures were reported with the following medications: vincristine, gadolinium, diatrizoate meglumine, doxorubicin, mercurochrome, paracetamol, and potassium chloride. These case reports confirm that misconnection events leading to wrong-route errors can occur and may cause serious injury. This comprehensive characterization of events was conducted to better inform clinicians and policymakers, and to describe an emergent strategy designed to mitigate patient risk.
- anesthesia
- local
- drug-related side effects and adverse reactions
- injections
- spinal
- nerve block
- neurotoxicity syndromes
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, an indication of whether changes were made, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- anesthesia
- local
- drug-related side effects and adverse reactions
- injections
- spinal
- nerve block
- neurotoxicity syndromes
Introduction
Neuraxial and peripheral analgesia are being used in a growing number of indications.1 2 Neuraxial anesthesia commonly supplements general anesthesia, and may serve as the primary anesthetic for surgeries conducted in the mediastinal region and lower extremities. It is applied in intraoperative, postoperative, and peripartum settings, as well as in end-of-life care. Increasingly, neuraxial routes are being used for acute pain therapy, chronic pain management, and diagnostic procedures. Peripheral nerve blocks provide pain control for patients undergoing surgery, and avoid the need for, and adverse effects linked to, opioids and volatile anesthetics.1 2 Given the range of neuraxial and peripheral applications, opportunities for human error are present.
Misconnection events can occur when delivery systems, such as tubes, syringes, or other accessories, are erroneously connected via an unintended route (eg, enteral, intravascular, neuraxial, or respiratory), leading to wrong-route medication administration.3–5 An example would be intravenous medication delivered to the epidural space.4 Misconnections leading to wrong-route medication delivery have been reported across a number of medical specialties, including anesthesiology, emergency medicine, obstetrics, and oncology.6–9 These events have occurred in a variety of settings, including inpatient surgery,10–14 labor and delivery,6 15 and intensive care units.16 17
Because of a lack of organized and publicly available reporting, it is not possible to assess the rate of misconnections wherein the denominator is the total number of catheter-based neuraxial and peripheral procedures.4 18–21 Several published reviews document that tubing misconnections are uncommon22–24; however, national research and hospital-based analyses confirm that misconnection events occur. For example, between January 2000 and December 2004, reports of more than 300 errors involving inappropriate intravenous/intravascular medication administration errors due to tubing interconnectivity were submitted to the US MEDMARX medication error-reporting database.22 Misconnections have also been reported by the Australian drug error database,24 a hospital in Japan,25 and tertiary care hospitals in South Africa.26
When these errors do occur, they exert a high burden in terms of morbidity and mortality, patient suffering, and expenses.27–29 In 2006, the Institute of Medicine estimated the average cost of managing any type of serious medication error to be US$8750 per preventable adverse drug event.30 These expenses can also include litigation costs and fines to hospitals, as well as increased length of stay and hospitalization costs.19 31–33
The goal of this narrative review is to characterize the current understanding of neuraxial and peripheral nervous system misconnection errors, and to motivate clinicians and health systems to consistently adopt the new International Organization for Standardization’s (ISO) standards.
Methods
This narrative review is based on a search of the medical literature and public access documents (English-language publications or abstracts) published between January 1, 1999, and September 30, 2019. Searches were conducted on PubMed and Embase using a Boolean strategy that initially applied US National Library of Medicine Medical Subject Headings (MeSH) terms to identify human cases; subsequently, a comprehensive sequence of MeSH and free-text terms were used to search indexed and non-indexed publications. These terms included: accidental, anaesth*/anesth*, ‘anesthetic incidents’, avoid*, blockade, bore, caudal, connector*, confus*, deadly, ‘drug administration routes’, drug error(s), ‘drug labeling’, ‘drug overdose’, epidural, erroneous*/error*, extradural, faulty, inadvertent infusion, injection, incident monitoring and reporting, interconnect*, intrathecal, intravenous, ‘Luer/Luer lock’, maladminist*, medication error(s), misconnect*, mistake*, mixup/mix-up(s), ‘mode of delivery’, ‘near-miss(es)’, ‘nerve block’, ‘non-Luer’, ‘organisational accidents’, peridural, peripheral, preventable, route, spinal, subarachnoid, syringe, ‘tube/tubing’, uninten*, ‘universal connector’, ‘universal design’, unknowingly, ventricular, ‘wrong *’, and ‘wrong route’. Similar terms were also applied to a general internet search for public access documents. See online supplemental table 1 for a full summary of literature search terms applied.
Supplemental material
The literature search was supplemented by bibliographic review of key source documents to identify case reports specific to wrong-route misconnection. Only case reports and other relevant publications that confirmed a misconnection event and noted the name of the drug administered and the patient’s health outcomes were included. Likewise, wrong-route cases attributed to causes other than misconnection (eg, mislabeled medication bags) were not included in the final analysis. Finally, to avoid reporting twice on the same case, source documents were reviewed closely to identify duplicate reports involving the same patient.
Once identified, cases were aggregated and descriptive analyses were conducted. The severity of each event was assessed using the National Reporting and Learning System incident criteria,23 as follows: (Low) Any unexpected or unintended incident that required extra observation or minor treatment and caused minimal harm to ≥1 person; (Moderate) Any unexpected or unintended incident that resulted in further treatment, possible surgical intervention, or canceling of treatment or transfer to another area, and which caused short-term harm to ≥1 person; (Severe) Any unexpected or unintended incident that caused permanent or long-term harm to ≥1 person; (Death) Any unexpected or unintended event that caused the death of ≥1 person. In addition, the frequency of reported misconnection/wrong-route events associated with specific drugs, and the drugs associated with high morbidity and mortality rates when administered by the wrong route, were evaluated.
Results
Approximately 200 unique publications were identified for full-text review; of these, 72 documents representing 133 case studies were determined to be relevant, and of these cases, 130 were included in the final case analysis (three were excluded owing to incomplete information regarding precise drug delivery route). Case study sources were published case reports and clinical studies (including journal manuscripts and scientific congress abstracts), as well as national policy alerts that included case study summaries. Case studies were identified from 25 countries and 6 continents (Africa, Asia, Australia/New Zealand, Europe, and North and South America) over a time period spanning from 1999 to 2019.
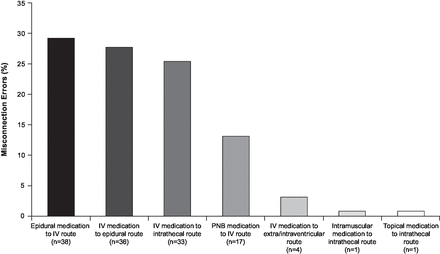
Of the reviewed documents, the most commonly reported neuraxial misconnection event type involved administration of medications intended for the epidural route by the intravenous route (29.2%, 38/130)11 25 26 34–41; a similar proportion of events involved the directionally opposite error (ie, administering medications intended for the intravenous route by the epidural route), at 27.7% (36/130)10 14 24–26 41–66 (figure 1). Medications intended for intravenous administration have been directly administered by the intrathecal route (25.4%, 33/130) due to misconnections.19 67–92 Less frequently, the misconnection error was the unintentional administration of a peripheral nerve block via the intravenous route (13.1%, 17/130). Two instances each have been described of injecting intravenous drugs into an intraventricular line13 or into an extraventricular drain.16 93 Least common were topical or intravascular medications administered by the intrathecal route, with one case each (0.77%).94 95 Although not a misconnection error, there have been instances of peripheral nerve block medications being administered on the wrong side of the body (7.9%, 10/126).24
Neuraxial and peripheral nerve block misconnection errors identified in case reports (N=130) between 1999 and 2019. Intended route unknown for 3 of 133 cases. IV, intravenous; PNB, peripheral nerve block.
The literature search identified 42 unique drugs or other substances injected via a misconnection event leading to wrong-route error; these misconnections were primarily neuraxial, but also included peripheral neural administration. Table 1 summarizes the 13 drugs with at least two published cases. Drug classes included: contrast agents,13 93 general anesthetics,47 57 60 64 local anesthetics,11 34–38 muscle relaxants,46 50 61 antifibrinolytics,81 92 nonselective adrenergic agonists,56 59 and nonsteroidal anti-inflammatory drugs.44 45 The greatest number of patient deaths were due to the erroneous administration of vincristine (n=15),19 68 73 75–78 80 82 84 87–89 96 thiocolchicoside (n=4),41 and bupivacaine (n=4).37 38 Moderate to severe harm has been caused by the erroneous epidural administration of potassium chloride49 52–54 62 and oxytocin.43 58 Detailed descriptions of the sequence of events in two cases of neuraxial misconnections are presented in online supplemental table 2).
Number, severity,* and route of drug administration errors identified in case reports: drugs with at least two case reports
For most drugs, or drug combinations, identified, there was only a single reported incident of accidental delivery; these drugs are listed in table 2.6 8 9 97 Among these cases, drugs came primarily from the following classes: antibiotics,10 42 72 90 chemotherapy agents,19 74 79 94 contrast agents,69 83 85 91 muscle relaxants other than thiocolchicoside,51 63 and opioids.42 55 71 Patient deaths were reported due to the erroneous intrathecal administration of the chemotherapy agent farmorubicin19 and tramadol.71 Other drug classes represented include beta blockers,70 acetylcholinesterase inhibitors,65 anticholinergics,65 decongestants, anticonvulsants,16 and cholinesterase inhibitors.86 Last, cases were reported involving the wrong-route administration of insulin,48 mercurochrome,95 parenteral nutrition,41 and sodium chloride.66
Individual drugs and other substances noted in a single case report involving neuraxial and peripheral misconnections, leading to wrong-route administration events
Discussion
This literature search identified published case reports describing neuraxial and peripheral misconnection events leading to wrong-route errors over a 20-year period. These events occurred across a range of healthcare settings and specialty areas and primarily involved the inadvertent delivery of drugs intended for intravenous administration via the intrathecal19 67–69 71 73 75–80 82 84 87–89 or epidural41 45 52 routes. Both death19 37 38 41 68 71 73 75–77 80 82 87 89 and serious morbidity (including paraplegia, paraparesis, spinal cord injury, and seizures)45 52 67 69 78 79 84 88 were associated with intravenous and neuraxial line misconnection errors. For most drugs identified, there was only a single reported incident of accidental delivery; this finding is similar to the results of previous literature reviews.6 8 9 97 In the most serious cases, outcomes were directly related to the toxicity of the drug that was inadvertently administered (eg, vincristine).
The case studies discussed in this narrative review support findings from several national monitoring systems that have evaluated the frequency of wrong-route misconnection errors. This research has consistently shown that these events occur, and that outcomes can be severe.22–24 27 98 For example, data from the US MEDMARX national error-reporting program and the Australian Incident Monitoring Study database suggest that between 1 in 25 and 1 in 250 intravenous-related medication errors are due to misconnections.22 24 Likewise, a retrospective analysis of the French Pharmacovigilance System found that 27 (96%)/28 cases of neuraxial wrong-route errors resulted in a serious adverse event, and 11 (39%)/28 resulted in patient death.27 However, these analyses relied primarily on self-reported information,22 24 27 making it impossible to calculate the true incidence of harmful outcomes. During the conduct of this literature review, we observed that the difficulties inherent in collecting error data, including errors that are not noticed or do not result in patient harm, add to the challenge of calculating true incidence. Of note, we observed that research attempting to identify all events using anonymized prospective reporting or observers documented higher rates of error and near-miss events26 39 99 than research that examined only voluntarily reported events.22–24
Universal Luer systems have long been used to securely connect fittings between needles, syringes, and tubing. These connectors serve multiple medication delivery routes, including intravenous, enteral, neuraxial, and respiratory.4 28 29 An unintended consequence of Luer standardization is an increased potential for patient harm or death due to misconnection between administration routes, leading to wrong-route medication administration.4 28 29 Reports of misconnection errors have led to efforts to create new, less error-prone systems. In 2010, the ISO 80369 series provided new standards to replace the universal use of Luer connectors.100 The ISO 80369 connectors introduce incompatibility between tubing connections serving different body systems and build in a safeguard to eliminate specific types of error. The ISO 80369-6 standard, introduced in 2016, specifies requirements for new Luer-incompatible neuraxial connectors; Luer connectors will continue to be used only for intravenous connections. Neuraxial applications include the administration of medications to neuraxial sites (ie, the spine, intrathecal space, ventricles of the brain, and the epidural, extradural, or peridural spaces), wound infiltration anesthesia delivery, other regional anesthesia procedures, and monitoring or removing cerebrospinal fluid for therapeutic or diagnostic purposes.101
Drug administration is the final step in a complex chain of events that includes procurement, storage, and deployment.102 In the hospital setting, there are several time points where medication error may occur, leading to a patient not receiving the right drug at the right time and the right place.22 Recognizing the relationship between Luer interconnectivity and medication error and adopting new ISO 80369 standards are critical steps in preventing future adverse outcomes.
Limitations
The data reported here are expected to be incomplete and are not intended to provide an estimate of incidence. This is because chronic under-reporting of misconnection events is widely acknowledged,4 18–20 96 103 and this literature search was confined to English-language cases. Furthermore, any interpretation of these data must be assessed in context with the relatively low real-world frequency of misconnection complications. When considering the very large number of regional anesthetic procedures performed worldwide, any potential safety intervention would require the treatment of large patient numbers before benefits could be documented. Therefore, it should be acknowledged that it may never be possible to track the true incidence of these events, or the global impact of any specific technologic intervention.
Conclusion
This literature review shows that misconnection events occur and can lead to wrong-route errors, with serious consequences. New technological advances are available that may improve patient safety by preventing misconnection events.
Acknowledgments
Assistance with manuscript preparation was provided to the authors by Caitlin Rothermel, MA, MPH of Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, USA, and was sponsored by Becton, Dickinson and Company, Franklin Lakes, NJ, USA.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors All authors participated in the planning, preparation, and critical review of this manuscript and granted final approval for submission.
Funding This study was funded by Becton, Dickinson and Company.
Competing interests FSS declares no conflicts of interest. VH and KH are employees of Becton, Dickinson and Company and own stock or stock options in the company. ERV receives consulting fees from AcelRx, Concentric, Heron Therapeutics, Innacoll, Merck, Neumentum, Pfizer, Recro, Salix, and Trevena.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data sharing not applicable as no datasets generated and/or analyzed for this study.