Article Text
Abstract
Background Postoperative pain after pediatric cardiac surgery is usually treated with intravenous opioids. Recently, the focus has been on postoperative regional analgesia with the introduction of ultrasound-guided erector spinae plane blocks (ESPBs). We hypothesized that bilateral ESPB with a programmed intermittent bolus (PIB) regimen decreases postoperative morphine consumption at 48 hours and improves analgesia in children who undergo cardiac surgery.
Methods This randomized, double-blind, placebo-controlled study comprised 50 children who underwent cardiac surgery through midline sternotomy. The patients were allocated randomly into two groups: ultrasound-guided bilateral ESPB at the level of T3–T4 transverse process then PIB with saline infusion (group 1, n=23) or PIB with 0.2% ropivacaine (group 2, n=27). Intravenous morphine at 30 µg/kg/hour was used as rescue analgesia. Postoperative pain was assessed using the COMFORT-B score for extubation, drain removal, and mobilization, and the FLACC (Face, Legs, Activity, Cry, Consolability) scale at 0, 2, 4, 6, 8, 12, 16, 20, 24, 36, and 48 hours after surgery. Adverse events were noted.
Results The total dose of morphine in 48 hours was significantly decreased in patients receiving a bilateral ESPB with ropivacaine (120±320 µg/kg) compared with patients with saline infusion (512±560 µg/kg; p=0.03). Fourteen per cent of patients required rescue analgesia with morphine in group 2 compared with 41% in group 1 (p=0.05). The patients in group 2 demonstrated significantly reduced COMFORT-B scores at extubation, drain removal, and mobilization compared with those in group 1 and had reduced FLACC scale levels at 20 and 24 hours postoperatively (p=0.05 and p=0.001, respectively). No differences were reported for extubation and drain removal times or for length of hospital stay. In addition, vomiting episodes were decreased in group 2 (p=0.01).
Conclusions In pediatric cardiac surgery, the results of this study confirm our hypothesis that bilateral ESPB analgesia with ropivacaine decreases the postoperative morphine consumption at 48 hours and demonstrates better postoperative analgesia compared with a control group.
Trial registration number NCT03593642.
- analgesics, opioid
- pain, postoperative
- regional anesthesia
- nerve block
Statistics from Altmetric.com
Introduction
Pediatric cardiac surgery is associated with moderate to severe postoperative pain that is related to median sternotomy, chest tubes, and costovertebral joint distension.1 Postoperative pain management after cardiac surgery is centered on intravenous opioids, which cannot provide complete pain relief and are responsible for side effects such as vomiting or respiratory depression.2 In fast-track programs, multimodal analgesia regimens including paracetamol, non-steroidal anti-inflammatory drugs, and opioids have been shown to be suboptimal after pediatric open cardiac surgery.3 Fifty-two per cent of pediatric patients reported moderate to severe pain on the day of surgery and 33% on day 1. In the same study, 42% of patients reported vomiting.3 Increasing interest has focused on opioid-sparing analgesic strategies to avoid some of the detrimental side effects of opioids.
Recently, the focus has been on postoperative regional analgesia after pediatric cardiac surgery.1 Thoracic epidural anesthesia or paravertebral blocks are efficacious but raise some concerns related to their potential complications.4 Several regional anesthetic techniques, including parasternal intercostal nerve blocks and serratus anterior plane block, have shown some potential to improve postoperative pain and decrease opioid consumption in the first 12 hours after surgery.5 6 Continuous infusions of local anesthetic (LA) into the sternal wound are under debate and do not demonstrate real improvement compared with placebo.7 8
Erector spinae plane block (ESPB)9 is an interfascial plane block whereby LA is injected beneath the iliocostalis, longissimus, and spinalis muscles to achieve multimetameric analgesia for pediatric thoracic, cardiac, or abdominal surgery.10–13 Its effect appears to be due to LA spread close to the paravertebral space, reaching the dorsal and ventral rami of the thoracic spinal nerves that cover dermatomes, which include the midline sternotomy. The primary goal of ESPB is optimally to achieve LA diffusion to the thoracic paravertebral space when the LA solution is located in between the erector spinae muscles and the paravertebral compartment.14
Recent studies reported that ultrasound-guided bilateral ESPB promoted good postoperative analgesia with reduced requirement for opioids as rescue analgesics in the 12-hour period after midline sternotomy in pediatric patients.15 There are few retrospective data10 16 concerning the first 48 hours postoperative period in terms of pain relief, opioid consumption, adverse events, or adaptations related to the continuous bolus technique and pharmacokinetics of LA injected in the erector spinae plane compartment.
In this double-blind, placebo-controlled study, we hypothesized that pediatric patients undergoing open cardiac surgery who received a programmed intermittent bolus (PIB) regimen for bilateral ESPB and opioid-sparing analgesia would be more likely to have decreased total opioid consumption in the 48 hours after surgery and experience improved postoperative pain relief compared with multimodal analgesia management without continuous ESPB. Adverse events associated with PIB ESPB and the pharmacokinetics of ropivacaine were also studied.
Patients and methods
Parents were given information about the study and written informed consent was obtained from all parents of the children included in the study. This randomized, double-blind, placebo-controlled study was performed from August 2018 to March 2019, initially with 104 children with an American Society of Anesthesiologists physical status class II scheduled for cardiac surgical procedures through midline sternotomy at Vinmec Central Park International Hospital, Ho Chi Minh City, Vietnam (figure 1). Exclusion criteria were as follows: the patient’s family refused, a preoperative ejection fraction <35%, ventricular arrhythmia/dysrhythmia, preoperative inotropic support, redo or emergency surgical procedures, and an allergy to amide-type LAs.
CONSORT diagram showing the flow of the study participants. CONSORT, Consolidated Standards of Reporting Trials; ESPB, erector spinae plane block; ICU, intensive care unit; PIB, programmed intermittent bolus.
Patients were enrolled by a physician blinded to the study and randomized (randomization number and the allocation group in a password-protected log file) into two groups using a computer-generated random number table: group 1 patients received an induction single-shot bilateral ESPB with 0.1%/0.2% ropivacaine followed by a PIB regimen for bilateral ESPB with saline for 48 hours, and group 2 patients received an induction single-shot bilateral ESPB with 0.1%/0.2% ropivacaine followed by a PIB regimen for bilateral ESPB with 0.1%/0.2% ropivacaine for 48 hours. Both groups received intravenous acetaminophen 15 mg/kg every 6 hours and ibuprofen 10 mg/kg every 12 hours as components of multimodal analgesia. Postoperative breakthrough pain episodes and moderate to severe levels of pain were managed with rescue intravenous morphine at 30 µg/kg/hour if necessary.
Anesthesia management
Midazolam 0.25 mg/kg was administered orally 2 hours before the patient was taken to the operating room. Standard monitoring for cardiac surgery anesthesia was applied with electrocardiography, pulse oximetry, end-tidal carbon dioxide, central temperature, urinary catheter, near-infrared spectroscopy, and entropy monitoring. Arterial line access was established for invasive blood pressure monitoring and a multilumen central venous line was inserted in the right internal jugular vein for central venous pressure monitoring and intravenous infusions. If necessary, transesophageal echography was applied for cardiac output and pressure monitoring. Antibiotic prophylaxis was achieved with cefazolin 30 mg/kg or vancomycin 15 mg/kg.
The children were induced with propofol at 2 mg/kg, sufentanil at 0.5–1 µg/kg, and rocuronium at 0.6 mg/kg as induction doses to facilitate endotracheal intubation. Anesthesia was maintained with sevoflurane (1%–2%) in 50% oxygen in air mixture, rocuronium at 0.4–0.6 mg/kg/hour, and sufentanil at 0.1–0.4 µg/kg/hour accordingly to maintain hemodynamic stability within 20% of the baseline value of arterial blood pressure from the sternotomy.
The ESPB procedure was performed in all patients as already reported,16 with the child in a right lateral decubitus position under aseptic conditions (figure 2A). A GE Logiq E ultrasound system with a 4–12 MgHz linear ultrasound transducer or a 15 MgHz hockey stick probe (GE Healthcare, Wauwatosa, Wisconsin) was used. The probe was placed in a longitudinal orientation over the T3 or T4 transverse process. After identifying the interfascial plane block beneath the iliocostalis, longissimus, spinalis, trapezius, and rhomboid major muscles, a 50 mm 20-gauge Tuohy needle (Perifix, B Braun, Melsungen, Germany) was inserted in-plane (figure 2B) and a 24-gauge epidural catheter was inserted in a cephalo-caudad direction. We aimed to place the tip of the catheter at the transverse process of T5 in the interfascial plane anterior to the erector spinae muscles, close to the intertransverse ligament (figure 2C). The catheter tip location at T5 permits to cover a metameric territory from T1 to T8 in infants and neonates (T8 entry point for drains). The catheter tip location was confirmed by hydrolocation with 0.5 mL of dextrose 5% indicating a solution spread in the fascial plane between the transverse process and erector spinae muscle.17 18 The catheters were secured with transparent dressings (figure 2D). In children <1 year, a bolus of 0.25 mg/kg/side of ropivacaine 0.1% was injected as an initial bolus, and in children >1 year an initial bolus of 0.50 mg/kg/side of ropivacaine 0.2% was injected. The procedure was repeated on the contralateral side. A maximum cumulative initial bolus of 6 mL (6–12 mg) per side was allowed.
(A) Procedure for ultrasound-guided erector spinae plane block. (B) Hyperechoic third and fourth thoracic vertebrae transverse process and above it the trapezius, rhomboid major, and erector spinae muscles from superficial to deep. The needle tip is located close to the third thoracic vertebrae transverse process. (C) The catheter is located in the interfascial plane anterior to the erector spinae muscles, close to the intertransverse ligament. The tip of the catheter is located at the T5 transverse process. (D) Dressings covering both catheters in a a 7-month-old boy. ESM, erector spinae muscle; RM, rhomboid major muscle; T, transverse process; TM, trapezius muscle.
After sternotomy, adequate heparinization (3 mg/kg intravenously and 25 mg in the bypass) was achieved with a resultant activated clotting time (ACT) >400 s before the initiation of cardiopulmonary bypass. On completion of cardiac repair, the patient was weaned off cardiopulmonary bypass followed by protamine administration (30 mg/kg) and ACT returned to baseline. After checking transfusion triggers, platelet count, prothrombin time, and blood gas values, the patients were transferred to a pediatric cardiac intensive care unit (ICU).
Postoperative ICU and postoperative pain management
The ESPB catheters were connected to a pump (Rythmic Evolution; Micrel Medical Devices, Gerakas, Greece) to deliver PIB. For postoperative analgesia, the patients in group 1 received 0.5 mL/kg/side PIB of saline every 6 hours (maximal volume 6 mL/side) and the patients in group 2 received 0.5 mL/kg/side PIB of ropivacaine 0.1%/0.2% every 6 hours (maximal volume 6 mL/side) into their ESPB catheters. The bolus on the second catheter was delayed by 1 hour in both groups.
All patients received intravenous paracetamol 15 mg/kg every 6 hours and ibuprofen 10 mg/kg every 12 hours. Postoperative pain assessment was performed by blinded research nurses using the COMFORT-B score19 in sedated patients in the ICU, after extubation, on first active mobilization, and at drain removal, and the FLACC (Face, Legs, Activity, Cry, Consolability) scale20 at 0, 2, 4, 6, 8, 10, 12, 16, 20, 24, 36, and 48 hours after surgery. For all patients, moderate to severe pain was defined according to a COMFORT-B score >17 or a FLACC scale value >3. The validity of these pain assessment tools and their cut-off point have been reported for treatment with analgesic medications/interventions.21 If pain persisted despite multimodal analgesia infusions, the patient was shifted to rescue analgesia using intravenous morphine 30 μg/kg/hour.
A twice-daily inspection of the catheter insertion points was done. If redness around the puncture point was noted, the catheter was removed and the patient changed to intravenous postoperative analgesia. Postoperative adverse effects such as nausea, vomiting, and cardiac arrythmia/dysrhythmia were recorded and treated.
Blood samples for analysis of plasma total ropivacaine concentration were taken from 10 patients in group 2 before the ESPB procedure, 1 hour after the induction dose of ropivacaine and at 48 hours. The blood sample before the ESPB is the control one; the 1-hour blood sample is considered as the maximal plasma concentration of ropivacaine related to systemic absorption of the initial bolus; the 48-hour blood sample is considered to be the plasma level of ropivacaine related to the cumulated dose of ropivacaine along the first 48 postoperative hours (duration of the study). A gas chromatograph was used as already reported.22 The limit of quantification for total ropivacaine concentration was 5 µg/L.
Standard monitoring was maintained in the ICU. The patients did not enter a fast-track program of extubation. A checklist for weaning and extubation criteria was used: conscious, plateau inspiratory pressure <15 cm H2O above positive end-expiratory pressure, a cardiac index >2 L/min m2 with dobutamine infusion <5 µg/kg min, a mean arterial pressure >50 mm Hg, urine output >0.5 mL/kg hour, no surgical bleeding with a hematocrit value >30%, hemoglobin level >100 g/L, normal coagulation, ionogram, and arterial blood gas analysis.
Statistical analysis
This study adheres to the applicable Consolidated Standards of Reporting Trials (CONSORT) guidelines. The total opioid consumption at 48 hours after surgery constituted the primary endpoint. The secondary endpoints included COMFORT score and FLACC scale value for postoperative pain in the 48 hours after surgery, the number of patients receiving morphine, the number free of intravenous morphine within the 48-hour period, the time to extubation and first alimentation, length of ICU stay, and incidence of adverse events. Considering the scant literature comparing the effectiveness of ultrasound-guided ESPB with a control group for postoperative analgesia in pediatric cardiac surgery, we based the sample size on our previous institutional unpublished retrospective data of morphine consumption in the first 48 hours after surgery in patients who received ultrasound-guided ESPB after open cardiac surgeries. In the control group of patients without ESPB, the mean (SD) consumption of intravenous morphine was 0.998 (0.323) mg/kg at 48 hours, and in the ESPB patients the mean consumption of intravenous morphine was 0.121 (0.035) mg/kg.
The aim of the present study was to demonstrate the effectiveness of ESPB by detecting an almost 30% difference in 48-hour total morphine consumption between the two groups of patients. The sample size was estimated at 18 patients per group (α=0.05; β=0.20 expected difference). We assumed up to 10% loss to follow-up and 10% of patients would be non-compliant; therefore, an estimated sample size of 22 children in each group was required. Categorical variables are expressed as number and percentage, and quantitative variables are expressed as median (IQR) or mean (SD). The Shapiro-Wilk test was used to test the normality of continuous variables. Univariate analysis was performed between continuous variables with the Student’s t-test or the Mann-Whitney test for non-Gaussian variables. Categorical variables were compared with the χ2 or Fisher’s exact test, as appropriate. A linear regression model with mixed effects was applied to include the impact of repeated measures in the same patients. A test was considered significant at p<0.05. Statistical analyses were performed using SAS V.11.
Results
The distribution and allocation of the patients are outlined in the CONSORT flow diagram (figure 1). One hundred and four children were initially recruited. Fifty-four were excluded at enrolment, hence 54 children were randomized and 50 were analyzed in the study. No differences in demographic characteristics, surgeries, intraoperative monitoring parameters, intraoperative sufentanil, and procedure duration were noted between the groups (table 1). Results for the primary and secondary endpoints are reported in table 2.
Demographic data, surgical procedures, and intraoperative variables
Comparison between groups for the primary and secondary endpoints
The use of a bilateral PIB ESPB with ropivacaine significantly decreased the amount of morphine used in the first postoperative 48 hours. Forty-one per cent of patients in the saline group received a total dose of 512±560 µg/kg of morphine in the first 48 hours compared with 14% of patients receiving a total dose of 120±320 µg/kg in the ropivacaine group (p=0.03). The results are reported in median (IQR), mean, and extreme values in figure 3. The OR with CI for receiving morphine in the first 48 hours in the control group is 3.7 (1 to 14.3) (p=0.05). Kaplan-Meier survival curves for time to event (figure 4) reported that there was a statistical difference in the proportion of patients who had not yet received intravenous morphine as rescue analgesia between the saline and the ropivacaine groups. Postoperative pain values evaluated by the COMFORT-B score at rest in sedated patients in the ICU, after extubation, at drain removal, and during the first active mobilization were significantly decreased in the ropivacaine PIB ESPB group (table 2).
Total dose of morphine received at 48 hours in both groups. For the box and whisker plots, the horizontal bar indicates the median, the upper and lower limits of the boxes indicate the IQR, and the ends of the whiskers are the 95% CI. Large circles are mean values. Small circles indicate extreme values. *P<0.05.
Kaplan-Meier survival curves for time to event for patients free of rescue analgesia with intravenous morphine.
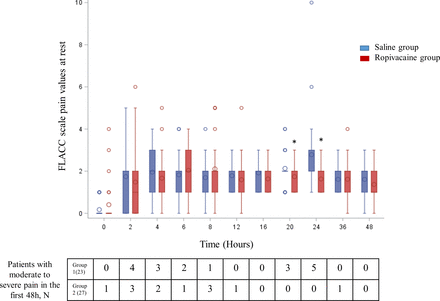
The trends for the FLACC scale values for postoperative pain at rest are depicted in figure 5; patients in the ropivacaine group reported significantly lower FLACC scores at 20 hours (p=0.05) and 24 hours (p=0.001) in comparison with patients in the saline group, with no significant difference at other times. No difference was reported for extubation time, time to drain removal, time to first active mobilization, and length of ICU and hospital stays (table 2). Table 3 reports the adverse events within the first 48 hours in both groups of patients. Vomiting episodes were decreased significantly in the ropivacaine PIB ESPB group (p=0.01). No significant difference was reported for the other reported adverse effects. Unfortunately 13% of patients in the saline group and 15% of patients in the ropivacaine group had involuntary catheter removal or displacement.
Pain scores reported with FLACC scale values. For the box and whisker plots, the horizontal bar indicates the median, the upper and lower limits of the boxes indicate the IQR, and the ends of the whiskers are the 95% CI. Circles indicate extreme values. *P<0.05. In the table are the numbers of patients with moderate to severe pain in the first 48 hours. FLACC, Face, Legs, Activity, Cry, Consolability.
Adverse events within the 48-hour postoperative period in both groups
The total ropivacaine concentration in plasma from 10 patients receiving PIB ESPB with ropivacaine was <0.005 µg/mL before the ESPB induction dose, with a mean±SD value of 0.16±0.13 µg/mL at 1 hour after the induction dose of ropivacaine and 0.46±0.49 µg/mL at the end of the 48-hour period of the PIB regimen.
Discussion
In this randomized, double-blind, placebo-controlled study, the use of bilateral ESPB with automated intermittent bolus of ropivacaine significantly reduced postoperative morphine consumption at 48 hours. Bilateral PIB ESPB with ropivacaine was effective in significantly relieving the postoperative pain values at 20 hours and 24 hours, and specifically after extubation and drain removal, with no effect on time to extubation and length of ICU/hospital stay. Furthermore, bilateral PIB ESPB using ropivacaine decreased the incidence of vomiting. Our study suggests that bilateral ESPB with a PIB infusion is a useful technique for opioid-sparing postoperative analgesic regimen after pediatric cardiac surgery.
Interest in perioperative regional anesthesia management was emphasized recently by Monahan et al,1 who reported that pediatric patients scheduled for open cardiac surgery may benefit from the addition of a variety of regional analgesia techniques. Regional analgesia-centered pain management after pediatric cardiac surgery is gaining popularity1 4–16 as a result of the decrease in morphine consumption reported in most recently published studies. In fast-track programs, multimodal analgesia regimens, including paracetamol and non-steroidal anti-inflammatory drugs without regional analgesia, have not been shown to be optimal for decreasing opioid consumption after pediatric open cardiac surgery.3 In patients not receiving a regional analgesia technique, the equivalent intravenous morphine use has been reported at median (range) doses of 102 (50–170) µg/kg in the postanesthesia care unit,3 and from mean±SD doses of 460±27 µg/kg in the first 12 hours postoperative period,15 from 400±90 µg/kg to 630±300 µg/kg at 24 hours,5 7 and 500±110 µg/kg at 48 hours.8 The consumption of intravenous opioids as rescue analgesics cannot provide full pain relief.
A recent study23 reported that only 43% of pediatric cardiac centers used regional anesthesia for intraoperative and postoperative analgesia. The most commonly applied technique was local infiltration anesthesia and single-shot paravertebral or intercostal blocks. Ultrasound-guided regional analgesic modalities demonstrated promising pain management and an opioid-sparing effect with single-shot injection.11 15 However, the scope of these studies focused only on the very early period after surgery. Kaushal et al 15 reported a significant decrease in postoperative consumption of fentanyl from extubation up to 12 hours in pediatric cardiac surgery patients receiving a bilateral single-shot ESPB with 1.5 mg/kg/side of 0.2% ropivacaine. Holland and Bosenberg11 noted low consumption of morphine at 70±40 µg/kg in patients with bilateral single-shot ESPB scheduled for pediatric thoracic and cardiac surgery in the early postoperative hours.
Studies on continuous wound infusion are debated.7 8 Few studies have reported that continuous ESPB techniques have an impact on morphine consumption at 48 hours.10 13 16 Our study reports that a bilateral PIB ESPB technique promotes a significant decrease in the number of patients requiring morphine rescue analgesia and a significant increase in patients free of intravenous morphine within the 48-hour postoperative period as well as a decrease in total intravenous morphine consumption in 48 hours. Our results corroborate those of Munshey et al,16 who reported that pediatric patients scheduled for thoracic or abdominal surgery with a PIB regimen for ESPB received a low median (IQR) intravenous morphine dose of 160 (150) µg/kg at 48 hours. Our patients used 120±320 µg/kg at 48 hours in the ropivacaine bilateral PIB ESPB group. No patient in the ropivacaine group complained about vomiting.
Our study reports that the bilateral PIB ESPB technique was effective for poststernotomy pain within a 48-hour period and particularly in painful situations (first mobilization, drain removal) compared with the control saline group. It has been reported that compared with systemic analgesia, regional anesthesia techniques reduce postoperative pain up to 24 hours in children undergoing cardiac surgery.1 Our results confirm those from previous studies11 15 16 reporting significantly lower pain scores until 12 hours after extubation in comparison with patients not receiving a bilateral ESPB or low mean±SD FLACC scale pain scores at 24 hours (1.7±1.8) and 48 hours (1.6±0.9) in patients undergoing thoracic surgery receiving automatic bolus ESPB.16 Our mean±SD values at 24 hours (1.63±0.6) and 48 hours (1.37±0.6) for the patients in group 2 are comparable. The significant difference between the two groups at 20 and 24 hours postoperatively is probably related to the end of the effect of the initial bolus of ropivacaine and the onset time for pain relief of rescue analgesics. That decrease is also reported in figure 4.
The bolus of LA solution administered in the ESPB catheter spreads cranially and caudally to reach multiple vertebral levels and the paravertebral space to anesthetize the dorsal and ventral rami of the spinal nerve roots, which results in analgesia of the ipsilateral hemithorax.24 The extent of the spread of the LA solution spread is still debated. Some ligament and layer structures are ventral to the erector spinae muscles. Intertransverse ligaments and superior costotransverse ligaments are found dorsal to the thoracic paravertebral space and constitute potential barriers to anesthetic solution spread. Those ligaments may be fenestrated, thereby permitting diffusion of LA molecules. Chin et al 9 reported that injection of 20 mL (0.3 mL/kg) of LA in adult patients produces clinical and radiological evidence of spread that extends at least three to four vertebral levels cranially and caudally from the site of injection and goes into epidural and paravertebral space. In the same way, Yang et al’s study25 reported that injection of a contrast dye resulted in reliable paravertebral spread. A sensory block of the anterior hemithorax26 clearly suggests that LA has successfully anesthetized the ventral ramus by penetrating the thoracic paravertebral space.27 These data may reinforce the results of our study concerning the postoperative pain relief related to sternotomy. However, a descriptive study28 refuted that hypothesis in patients receiving a PIB injection of 15 mL of 0.2% levobupivacaine in whom four segments were anesthetized at the anterior axillary and midclavicular line, whereas no hypoesthesia was detected in the parasternal region. In adults there is still a debate on the fact that the injectate spreads to the back muscles and fascial layers predominantly compared with the extent of paravertebral spread.29
In pediatric patients, the anatomy and physiology of ESPB look different. A recent study18 of two neonatal cadavers supports the fact that 0.1 mL/kg of LA is required for each dermatome in preterm neonates. Craniocaudal spread was noted from vertebral levels T3–T6 when 0.5 mL of dye was introduced at T5 and was found anteriorly in the paravertebral and epidural spaces, in the area of both the dorsal and ventral rami of the spinal nerves T2–T12. These results are confirmed by another study that reported in neonatal cadavers a contrast dye spread over three to four vertebral levels in the paravertebral space.30 We used a PIB regimen for ESPB analgesia in our study. The PIB regimen allowed for larger volumes of LA than a continuous infusion, possibly allowing for more constant spread of LA solution through the intervertebral foramina and interfascial compartments. The volume of 0.2% ropivacaine we used in children >1 year was 0.5 mL/kg for PIB that was in the range of the volumes used in the pediatric ESPB literature. Holland and Bosenberg11 reported a mean volume of 0.4±0.17 mL/kg on each side, Munshey et al 16 a volume of 0.3 mL/kg/hour and Kaushal et al 15 a volume of 0.75 mL/kg. The dose of ropivacaine used for induction or bolus in the present study is in accordance with the recent literature evaluating regional anesthesia in pediatrics.31
The other area of interest concerning the use of ESPB is the reduction in adverse events related to the postoperative use of opioids or more invasive regional anesthesia techniques. Opiates are responsible for various side effects, such as vomiting, in 18%–42% of patients,1 3 or respiratory depression.2 We noted that 17% of patients receiving saline complained about vomiting, but 0% of patients in the ropivacaine group. The use of PIB with low doses of ropivacaine limits the risk of LA toxicity. Moreover, for the bilateral erector spinae plane catheters, we decided to alternate the bolus by 1 hour into the catheters, avoiding toxic doses. The plasma concentrations of ropivacaine at the times taken, that is, at 1 hour and 48 hours, were below the recognized toxic level. The plasma ropivacaine value that is considered safe in all situations is reported at 1.5 µg/mL and the authorized maximal plasma level at 2.2 µg/mL.32
There were a few limitations to the study. First, the sample size was relatively small. Second, we did not enter the pediatric patients into a fast-track anesthesia with on-table extubation program, which could be the best situation for the use of bilateral ESPB analgesia. However, rapid extubation process is performed regularly in only 26% of pediatric patients scheduled for cardiac surgery23 and is complicated by a 48-hour reintubation rate in 12% of cases.33 The use of an initial bolus of ropivacaine in both groups has probably masked the expected difference concerning time to extubation or length ICU stay. Third, we did not assess the dermatomal sensory blockade in our patients.
In conclusion, this study showed that in pediatric open cardiac surgery, ultrasound-guided bilateral ESPB analgesia with ropivacaine produced a significant decrease in opioid consumption at 48 hours postoperatively, including decreased access to opioids for patients, and demonstrated better postoperative analgesia compared with a control group.
References
Footnotes
Contributors PM was involved in the study design, performing the procedures, analyzing the data, and writing the paper. NH and VN provided the main contribution of performing the procedures and analyzing the data. HPV contributed to the study with plasma ropivacaine dosages. KDNT contributed to the study with the plasma ropivacaine dosages and analyzing the data. SB helped with writing the paper and performing the statistical analysis and methodology. XC was involved in analyzing the data, writing the paper, and editing the final version.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval The study was approved by Vinmec International Hospital JSC institutional ethical committee (QD-VMEC133/128.05.2018).
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.