Article Text
Abstract
Background and objectives There is a need for local anesthetics that provide consistent analgesia through 72 hours after surgery. This study evaluates the use of HTX-011 (bupivacaine and meloxicam in Biochronomerpolymer technology), an extended-release, dual-acting local anesthetic, in reducing both postoperative pain over 72 hours and postoperative opioid use when compared with bupivacaine hydrochloride (HCl) and saline placebo. Inclusion of low-dose meloxicam in HTX-011 is designed to reduce local inflammation caused by surgery, potentiating the analgesic effect of bupivacaine. Previously, significant synergy has been observed with bupivacaine and meloxicam with both given locally together.
Methods EPOCH 1 was a randomized, double-blind, placebo-controlled and active-controlled phase III study in subjects undergoing a primary unilateral, distal, first metatarsal bunionectomy in which subjects received either a single intraoperative dose of HTX-011, immediate-release bupivacaine HCl or saline placebo.
Results A total of 412 subjects were dosed. The results for the primary and all four key secondary endpoints were statistically significant in favor of HTX-011. HTX-011 demonstrated superior, sustained pain reduction through 72 hours, significantly reduced opioid consumption and resulted in significantly more opioid-free subjects compared with saline placebo and bupivacaine HCl. Safety was similar across groups with fewer opioid-related adverse events observed in the HTX-011 group.
Conclusions HTX-011 demonstrated significant reduction in postoperative pain through 72 hours with significant reduction in opioid consumption and a significant increase in the proportion of opioid-free subjects compared with saline placebo and the most widely used local anesthetic, bupivacaine HCl.
Trial registration number NCT03295721.
- lower extremity
- postoperative pain
- interventional pain management
- pain medicine
This is an Open Access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, an indication of whether changes were made, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
More than 500 000 bunionectomy procedures are performed in the USA annually, making it one of the most common surgeries. Patients most often experience the greatest degree of pain within the first 72 hours after surgery, with the majority experiencing moderate to severe pain.1–5 Perioperative administration of local anesthetics such as bupivacaine is commonly used in clinical practice for managing postoperative pain; however, these agents have shown limited efficacy beyond 6–12 hours, necessitating the use of additional postoperative analgesics, including opioids.6–8 Extended-release liposomal bupivacaine can potentially extend the period of pain relief but rarely beyond 24 hours; it has not shown superiority to bupivacaine hydrochloride (HCl) in bunionectomy.6–8 There is a need for local anesthetics that can provide consistent analgesia through the first 72 hours when patients experience the most severe pain. Opioids are commonly used for postoperative pain management. The use of opioids is associated with an increased risk of adverse drug effects including respiratory depression, sedation, and postoperative nausea and vomiting.9 10 Across surgical models, poorly managed pain and opioid-related adverse events (ORAEs) have been shown to contribute directly to patient discomfort, dissatisfaction, delayed recovery from surgery, increased length of hospital stay and increased medical costs.11–14
HTX-011 is a novel, extended-release, dual-acting local anesthetic that is a fixed-dose combination of two active ingredients, bupivacaine and low-dose meloxicam, incorporated in a proprietary Biochronomer polymer. The product is a viscous solution administered directly into the surgical incision via a syringe with a Luer lock applicator to coat the affected tissue prior to suturing. After single-dose administration, the polymer enables extended release of bupivacaine and meloxicam simultaneously over approximately 3 days. Inclusion of low-dose meloxicam in HTX-011 reduces local inflammation caused by surgery and normalizes the local pH,15 16 allowing the analgesic effect of bupivacaine. This synergistic relationship has been demonstrated in prior phase II studies in bunionectomy and herniorrhaphy, where HTX-011 led to a substantially greater reduction in pain intensity when compared with the same polymer formulation containing either bupivacaine alone or meloxicam alone.17
The objectives of this randomized, double-blinded, placebo-controlled and active-controlled phase III study (EPOCH 1) were to evaluate the analgesic efficacy and safety of HTX-011 at a single dose of 60 mg/1.8 mg applied into the surgical site without a needle, compared with bupivacaine HCl injection (the most widely used local anesthetic for addressing postoperative analgesia) and saline placebo in subjects undergoing a primary unilateral, distal, first metatarsal bunionectomy with osteotomy and internal fixation.
Methods
Study design
The study was conducted at 13 sites across the USA from October 2017 to March 2018. All subjects provided written informed consent prior to participation in any study-specific procedures.
Eligible subjects were required to be at least 18 years of age with an American Society of Anesthesiologists physical status of I, II or III. The study excluded subjects with a pre-existing, concurrent acute or chronic painful physical/restrictive condition that could confound the postoperative assessments. Other key exclusion criteria included the use of non-steroidal anti-inflammatory drugs (NSAIDs; including meloxicam) within 10 days prior to the scheduled surgery, known or suspected daily use of opioids for seven or more consecutive days within 6 months prior to surgery, long-acting opioids within 3 days prior to surgery, the use of any opioids within 24 hours prior to surgery, the administration of bupivacaine within 5 days prior to surgery and the use of systemic steroids within five half-lives or 10 days prior to administration of study drug.
Given the double-blind study design, neither the subjects nor the investigators involved in conducting postsurgical assessments knew which treatment was given. Subjects were randomly assigned using a centralized computer-generated blocked randomization algorithm in a 3:3:2 ratio to three treatment groups: (A) HTX-011, 60 mg/1.8 mg (bupivacaine/meloxicam), 2.1 mL, applied into the surgical site without a needle; (B) bupivacaine HCl 0.5%, 50 mg (10 mL), via injection into the surgical site; and (C) saline placebo, 2.1 mL, applied into the surgical site without a needle. On day 1, the day of surgery, subjects underwent a unilateral simple Austin-type bunionectomy under regional anesthesia with no more than 20 mL of 1% lidocaine without epinephrine administered as a Mayo block. Epidural or spinal anesthesia was not permitted. During surgery, the use of opioids (other than intravenous fentanyl) or other analgesics was prohibited.
Near the end of surgery, after irrigation and suction, a single dose of study drug (HTX-011, bupivacaine HCl or saline placebo) was administered intraoperatively via local application into the surgical site by the investigator. Subjects remained in the hospital/research facility for a minimum of 72 hours following surgery to undergo postoperative assessments including pain intensity, opioid use and collection of blood samples for assessment of pharmacokinetics. Subjects could only receive rescue medication on request to treat postoperative pain, not for pain prophylaxis, during the 72 hours postoperative observation period. Postoperative rescue medication consisted of intravenous morphine (no more than 10 mg within a 2-hour period as needed), oral oxycodone (no more than 10 mg within a 4-hour period as needed) and/or oral acetaminophen (no more than 1000 mg in a 6-hour window). No other analgesic agents were permitted. After the 72 hours, assessments were completed, subjects could be discharged and were instructed to return to the study site on day 10, day 28 and day 42 to complete follow-up assessments. After discharge through day 28, subjects were to complete a daily diary to record whether they took opioids.
Outcome measures
The primary efficacy endpoint was mean area under the curve (AUC) of the Numeric Rating Scale (NRS) of pain intensity scores through 72 hours (AUC0-72) for HTX-011 compared with saline placebo. During the first 72 hours following surgery, the NRS was measured at hours 1, 2, 4, 8, 12, 24, 36, 48, 60 and 72. Four key secondary efficacy endpoints were: (1) mean AUC0-72 of the NRS pain intensity scores for HTX-011 compared with bupivacaine HCl, (2) mean total postoperative opioid consumption (in morphine milligram equivalents) through 72 hours for HTX-011 compared with saline placebo, (3) the proportion of subjects who were opioid free through 72 hours for HTX-011 compared with bupivacaine HCl and (4) the mean total postoperative opioid consumption (in morphine equivalents) through 72 hours for HTX-011 compared with bupivacaine HCl. To account for multiple hypothesis testing on the primary endpoint and on each of the four key secondary endpoints, a strict testing hierarchy was applied to control study-wise alpha level at 0.05. Other secondary efficacy endpoints included the proportion of subjects with severe pain (defined as an NRS pain intensity score ≥7 at any timepoint through 72 hours) and the proportion of subjects who were opioid free through 72 hours compared with placebo, through day 10 and through day 28.
Safety endpoints included the incidence of treatment-emergent adverse events (TEAEs), change from baseline in clinical laboratory results, ECG, vital signs, wound healing at 72 hours and 10, 28 and 42 days after treatment, bone-healing X-ray assessments at 28 and 42 days after treatment and ORAEs. ORAEs were based on prespecified preferred adverse event (AE) terms of nausea, vomiting, constipation, pruritus, pruritus generalized, somnolence, respiratory depression and urinary retention, regardless of whether a subject actually received an opioid medication.
Statistical analysis
Based on prior phase II studies of HTX-011 in subjects undergoing unilateral simple bunionectomy, a sample size of approximately 400 subjects (150, 150 and 100 in HTX-011, bupivacaine HCl and saline placebo groups, respectively) was needed to provide at least 90% power to detect a statistically significant difference between the HTX-011 group and the control groups for each of the primary and key secondary endpoints.
The primary and first key secondary endpoints were analyzed using analysis of variance with treatment group as the main effect, together with pairwise t tests to analyze differences between treatment groups. Missing data, which were expected to be very low due to the 72 hours hospitalization following surgery, were imputed via last observation carried forward for interval censored pain intensity scores and worst observation carried forward (WOCF) for right-censored pain intensity scores in the intent-to-treat (ITT) population. To adjust for the analgesic effect of opioid rescue medication, the windowed WOCF method was implemented as the primary analysis method in which pain intensity scores observed during the analgesic window of any opioid rescue medication were replaced with the worst postdose, non-missing NRS pain intensity score observed prior to the rescue medication window. A sensitivity analysis of the primary endpoint was performed with no adjustment for opioid usage. The total postoperative opioid consumption through 72 hours was analyzed using a Wilcoxon rank-sum test. The proportion of subjects who were opioid free through 72 hours was analyzed using Fisher’s exact test. All TEAEs were coded and tabulated by System Organ Class and Preferred Term.
Results
Baseline characteristics
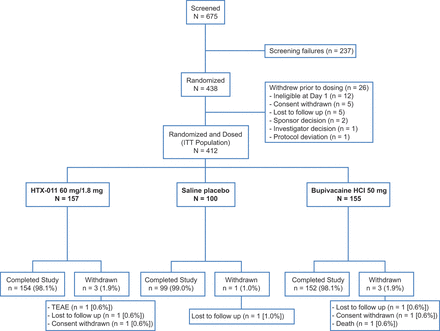
Of 438 subjects who were randomly assigned to the three study groups, 412 received one of the three interventions (ITT population; figure 1). Baseline disease characteristics were similar between groups and most of the subjects were female, as expected given the surgical procedure (table 1).3
Baseline demographic by study group (ITT population)*
Consort flow diagram for EPOCH 1 study. HCl, hydrochloride; ITT, intent to treat; TEAE, treatment-emergent adverse event.
Efficacy findings: primary and secondary endpoints
HTX-011 demonstrated statistically significant as well as clinically relevant benefit for the primary endpoint and all four key secondary endpoints (table 2). Compared with saline placebo and bupivacaine HCl, application of HTX-011 resulted in sustained pain reduction through 72 hours, significantly less opioid consumption and significantly more subjects who remained opioid free.
Efficacy results for the primary and key secondary endpoints (ITT population)*
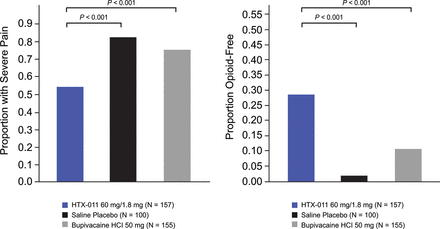
Subjects who received HTX-011 showed a reduction in mean pain intensity over 72 hours of 27% compared with saline placebo (323.3 vs 445.3; p<0.001; the primary endpoint) and 18% compared with bupivacaine HCl (323.3 vs 393.5; p<0.001; the first key secondary endpoint). Compared with saline placebo, mean NRS pain intensity scores were lower in the HTX-011 group at all timepoints through 72 hours. A prespecified sensitivity analysis with no adjustment of opioid use consistently showed significant pain reduction for HTX-011 compared with bupivacaine HCl and saline placebo (p<0.01 for both comparisons), confirming the robustness of the results of the primary analysis. Over the course of 72 hours after treatment, total opioid consumption was significantly reduced by 37% in those who received HTX-011 when compared with saline placebo (p<0.001; the second key secondary endpoint) and by 25% vs those who received bupivacaine HCl (p=0.002; the fourth key secondary endpoint). Overall, 29% of subjects who received HTX-011 were opioid free after 72 hours, whereas 11% of those who received bupivacaine HCl (p<0.001; the third key secondary endpoint) and 2% of those who received placebo (p<0.001) were opioid free (figure 2). In addition, a significantly greater proportion of subjects who received HTX-011 were able to go without any rescue medication, including acetaminophen. After 72 hours, those treated with HTX-011 remained opioid free at a significantly higher rate through day 10 and through day 28 compared with subjects who received saline placebo or bupivacaine HCl. Among the 45 HTX-011 subjects who were opioid free during the 72 hours after surgery, 41 (91.1%) stayed opioid free though day 10 and 37 (82.2%) stayed opioid free though day 28.
Proportion of subjects experiencing severe pain at any time from 0 to 72 hours and proportion of subjects opioid free through 72 hours (ITT population). HCl, hydrochloride; ITT, intent to treat.
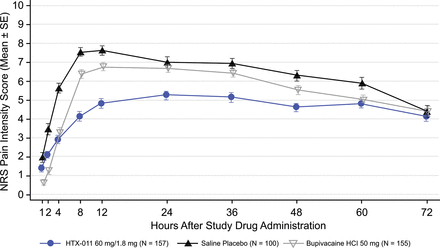
A separation in mean pain curves through 72 hours was apparent between groups who received HTX-011, bupivacaine HCl and saline placebo (figure 3). Fewer subjects in the HTX-011 group experienced severe pain (53.5%); this proportion was significantly lower compared with saline placebo (83.0%) and bupivacaine HCl (75.5%; p<0.001 for both comparisons) (figure 3).
Mean (SE) NRS pain intensity scores using wWOCF (ITT population). HCl, hydrochloride; ITT, intent to treat; NRS, Numeric Rating Scale; wWOCF, windowed worst observation carried forward.
Subjects who received bupivacaine HCl experienced significantly lower pain in the first 12 and 24 hours after surgery (AUC0-12 and AUC0-24) compared with those who received saline placebo, but pain in those who received bupivacaine HCl was not significantly different from placebo beyond 24 hours as measured by AUC24-72 (table 3, figure 3).
Mean AUC of th NRS of pain intensity over time using wWOCF (ITT population)*
By comparison, HTX-011 significantly reduced pain in the first 12 and 24 hours versus both saline placebo and bupivacaine HCl (AUC0-12 and AUC0-24; p<0.001 for both comparators), and this reduction was maintained beyond 24 hours through the full 72 hours period (AUC24-72; p<0.01 vs bupivacaine HCl; p<0.001 vs placebo).
Safety
HTX-011 was well tolerated and exhibited a TEAE profile like that of saline placebo and bupivacaine HCl (table 4).
Overall summary of TEAE (safety population)*
The two most common TEAEs in the HTX-011 group were nausea and dizziness. The incidence of nausea was numerically lower in the HTX-011 group compared with both the saline placebo and bupivacaine HCl group (37.6% vs 43.6% and 45.5%, respectively). There were no differences in serious adverse events between groups, no drug-related serious adverse events and no discontinuations due to drug-related adverse events in the HTX-011-treated subjects. One death of unknown cause occurred 17 days after a subject received bupivacaine HCl and was deemed unlikely related to study drug. There was no evidence of drug-related local anesthetic systemic toxicity (LAST) based on a comprehensive review of TEAEs, vital signs, ECGs and bupivacaine plasma concentrations (highest concentration observed was 190 ng/mL). The proportion of subjects reported to have any wound-healing findings was similar across treatment groups. The most common wound-healing findings across all treatment groups were bruising, erythema and edema, with most resolving by the day 42 safety follow-up visit. There was no evidence of drug-related delayed bone healing based on X-rays through day 42 for all treatment groups. A lower proportion of subjects experienced ORAEs in the HTX-011 group compared with the saline placebo and bupivacaine HCl groups (43.9% vs 53.5% and 50.6%, respectively). No clinically meaningful differences were observed between HTX-011, saline placebo and bupivacaine HCl for other tested safety parameters such as hematology and serum chemistry, vital signs and ECGs.
Discussion
In this phase III study, HTX-011 provided superior and sustained pain reduction compared with bupivacaine HCl through the critical 72 hours postoperative window, when pain is often most severe. HTX-011 is the only local anesthetic that has demonstrated such efficacy in a phase III study. Significant reductions in pain occurred both early (in the first 24 hours) and were sustained from 24 hours to 72 hours. In addition to reductions in average pain scores, HTX-011 significantly reduced the proportion of patients ever experiencing severe pain through 72 hours. The significant reductions in severe pain were consistent with the significant increase in opioid-free subjects over that period, and most subjects that required no opioids in the first 72 hours continued to be opioid free through 28 days.
Bupivacaine HCl solution administered by standard infiltration technique was chosen as the active comparator in this study because it accounts for approximately 70% of all local anesthetic usage in the USA (data on file). This study was powered to demonstrate head-to-head superiority of HTX-011 60 mg/1.8 mg over bupivacaine HCl 50 mg using a statistically rigorous gated approach. The finding that bupivacaine HCl significantly reduced pain in the first 12 and 24 hours versus placebo confirmed that the selected 50 mg dose was appropriate. The study also confirmed that bupivacaine HCl does not produce significant pain reduction beyond 24 hours. Conversely, HTX-011 produced superior pain reduction versus bupivacaine HCl and placebo through the first 12 and 24 hours and beyond 24 hours through 72. These results were shown to be robust when a prespecified sensitivity analyses without adjustment of opioid analgesic effect also showed superiority of HTX-011. This indicates that the statistical significance of the primary efficacy outcome was not driven by the adjustment for opioid rescue medication use. When not adjusting pain scores for opioid rescue medication use, between-group differences remained significant, even though considerably more subjects in the saline placebo and bupivacaine HCl control groups than in the HTX-011 groups received opioid rescue medication and the total opioid consumption was significantly higher in the control groups. This is the first time, to our knowledge, that an extended-release local anesthetic has shown significant analgesia versus either placebo or bupivacaine HCl beyond the first day after a single administration with or without adjustment for opioid use.
The 60 mg dose level for bupivacaine in HTX-011 was used based on prior phase II experience in bunionectomy.17 It is notable that approximately a third of the bupivacaine (22 mg) is released over the first 24 hours at this dose from the HTX-011 and the very low dose of meloxicam in HTX-011 has been shown to produce no direct analgesic effect (data on file). Therefore, the superiority of HTX-011 60 mg/1.8 mg (bupivacaine/meloxicam) to bupivacaine HCl 50 mg is most likely because of synergy of the dual-acting components in HTX-011, as was previously observed in phase II studies.17
The clinical benefits of HTX-011 treatment included significantly lowering the proportion of subjects who experienced severe pain, resulting in almost 30% more HTX-011-treated subjects not requiring any opioids during the 72-hour period compared with placebo. Consistent with fewer subjects taking any opioids, HTX-011-treated subjects experienced fewer ORAEs. Furthermore, among the HTX-011-treated subjects who were opioid free through 72 hours, more than 90% remained opioid free through day 10 and 82% remained opioid free through day 28. These results may have further clinical relevance given that higher levels of opioid use in the acute postoperative period have consistently been found to predict chronic opioid use months after surgery.18–20
HTX-011 was well tolerated, with an AEs profile like that of saline placebo and bupivacaine HCl. Systemic toxicities with local anesthetics have been associated with high bupivacaine blood concentrations. In this study, there was no evidence of LAST with HTX-011. There was no evidence of delayed bone healing in this study, which included multiple assessments of bone healing and is consistent with several studies showing that short-term use of low-dose NSAIDs does not interfere with bone healing.21–23
This study has some limitations. Subjects were kept in-hospital for 72 hours rather than discharged at 1–2 hours in order to ensure robust pain and pharmacokinetic data collection. Since there are no standard doses for bupivacaine HCl in bunionectomy, the dose selected for the active control group was based on guidance from experienced surgeons and was within bupivacaine HCl labeling; statistically significant reductions in pain through 24 hours compared with placebo were observed for bupivacaine HCl, confirming the appropriateness of the selected dose. Although the current study focused on subjects undergoing bunionectomy, this is a well-established bony pain model,3 5 and therefore, the results may be generally applicable to other surgical procedures. Although this trial did not use scheduled multimodal analgesics (in order to assess the benefit of HTX-011 alone postoperatively for regulatory requirements), addition of non-opioid analgesics would be expected to further improve pain control and reduce opioid utilization. Future studies incorporating other non-opioid agents are necessary to determine whether HTX-011 can serve as the keystone of non-opioid pain protocol for patients undergoing foot and ankle procedures.
In summary, the first 72 hours after surgery, when pain is the most severe, is also the most crucial for pain management and optimal patient recovery. Effective pain management and reduced exposure to opioids are associated with improved patient outcomes and reduced risk for ORAEs and the development of chronic opioid use. HTX-011, a dual acting local anesthetic, is the first locally administered product to demonstrate superior, sustained pain reduction for 72 hours compared with both saline placebo and bupivacaine. Furthermore, the superior pain reduction with HTX-011 resulted in significantly fewer subjects experiencing severe pain, leading to reduced opioid consumption and a lower proportion of subjects who experienced ORAEs. Importantly, a significantly higher proportion of subjects receiving HTX-011 had a completely opioid-free recovery following surgery. In light of the current need within the USA to reduce the amount of opioids prescribed to postsurgical patients, significant increases in the proportion of opioid-free patients may enable physicians to write fewer of these prescriptions. HTX-011 was well tolerated with an AE profile similar to saline placebo and bupivicaine.
Acknowledgments
We would like to thank the patients for their participation in this phase III trial, the clinical trial site staff members, trial coordinators, statisticians, supporting staff and remaining members of the trial teams. Funding for this research was provided by Heron Therapeutics, Inc (San Diego, California). Assistance with manuscript preparation was provided by Chung H Lou at Samorn Biosciences (San Diego, California) and by Alexis Fedorchak, PhD and Andrew Occiano, PharmD at ApotheCom (San Francisco, California) and was funded by Heron Therapeutics, Inc (San Diego, California).
References
Footnotes
Contributors The authors conceived of and designed the study, analyzed and interpreted the data, drafted the manuscript and provided critical revisions to the manuscript.
Funding This study was supported by Heron Therapeutics, Inc.
Competing interests EV receives research grants from Pacira and Durect and receives consulting fees from AcelRx, Avenue, Cara, Concentric, Heron Therapeutics, Innacoll, Mallinckrodt, Merck, Neumentum, Pacira, Pfizer, Recro, Salix and Trevena; JSG has nothing to disclose; G-CL and RAP receive consulting fees from Heron Therapeutics; JH is an employee of Heron Therapeutics and receives salary and stock options.
Patient consent for publication Not required.
Ethics approval The study protocol was approved by a centralized institutional review board (Aspire IRB, Santee, California).
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available on reasonable request.