Abstract
Patients with moderate-to-severe malignancy-related pain require opioid pharmacotherapy. Many cancer patients continue to be prescribed subtherapeutic doses of pain medications resulting in undue suffering and diminished quality of life. Pain associated with malignancy and its treatment may exacerbate other symptoms associated with cancer, including nausea, fatigue, weakness, dyspnoea, constipation and impaired cognition. The choice of analgesic pharmacotherapy should be individualised and based on the intensity of pain reported by the patient, rather than its specific aetiology. When selecting pain management pharmacotherapy, the healthcare provider should consider the patient’s pain level, activity level and any comorbid illness. Intolerable adverse effects, ineffective pain relief or a change in the patient’s clinical status can dictate the need for a new pain management regimen.
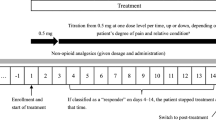
Healthcare providers must be able to readily quantify the relative analgesic potency when converting from one opioid to another or from one route of administration to another. Transdermal formulations of fentanyl and buprenorphine are effective pharmacotherapy that can be safely used for cancer patients with pain. However, clinicians need to be cognisant that the US/UK manufacturer’s recommendations for equianalgesic dose administration of transdermal fentanyl may result in initial doses that produce subtherapeutic concentrations and unrelieved pain in some patients. A less conservative dose administration algorithm for transdermal fentanyl using a 2: 1 (mg/day of oral morphine: μg/h of transdermal fentanyl) conversion ratio that considers both a review of the literature and clinical experience should help clinicians individualise cancer pain pharmacotherapy.






Similar content being viewed by others
References
Cleeland CS, Gonin R, Hatfield AK, et al. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med 1994; 330(9): 592–6
Coyle N, Adelhardt J, Foley KM, et al. Character of terminal illness in the advanced cancer patient: pain and other symptoms during the last 4 weeks of life. J Pain Symptom Manage 1990; 5(5): 83–93
Grond S, Zech D, Diefenbach C, et al. Prevalence and pattern of symptoms in patients with cancer pain: a prospective evaluation of 1635 cancer patients referred to a pain clinic. J Pain Symptom Manage 1994; 9(6): 372–82
Simmonds MA. Pharmacotherapeutic management of cancer pain: current practice. Semin Oncol 1997; 24 (5 Suppl. 16): S16.1-16.6
Zhukovsky DS, Goroswski E, Hausdorff J,et al. Unmet analgesic needs in cancer patients. J Pain Symptom Manage 1995; 10(2): 113–9
Ferrer-Brechner T. The management of pain associated with malignancy. Semin Anesth 1985; 4: 313–22
Bucher JA, Trostle GB, Moore M. Family reports of cancer pain, pain relief, and prescription access. Cancer Pract 1999; 7(2): 71–7
Hanks GW. Cancer pain and the importance of its control. Anticancer Drugs 1995; 6 Suppl. 3: 14–7
World Health Organization. Cancer pain relief: with a guide to opioid availability. 2nd ed. Geneva: WHO, 1996
Zech DF, Grond SA, Lunch J,et al. Validation of World Health Organization guidelines for cancer pain relief: a 10-year prospective study. Pain 1995; 63(1): 65–76
Schug SA, Merry AF, Acland RH. Treatment principles for the use of opioids in pain of nonmalignant origin. Drugs 1991; 42(2): 228–39
Zenz M, Strumpt M, Tryba M. Long-term oral opioid therapy in patients with chronic nonmalignant pain. J Pain Symptom Manage 1992; 7(2): 69–77
Portenoy RK. Opioid therapy for chronic nonmalignant pain: a review of the critical issues. J Pain Symptom Manage 1996; 11(4): 203–17
Arner S, Meyerson BA. Lack of analgesic effect of opioids on neuropathic and idiopathic forms of pain. Pain 1988; 33(1): 11–23
Foley KM. The treatment of cancer pain. N Engl J Med 1985; 313(2): 84–95
Cherny NI, Portenoy RK. Cancer pain management: current strategy. Cancer 1993; 72 (11 Suppl.): 3393–415
American Pain Society. Principles of analgesic use in the treatment of acute pain and chronic pain. 5th ed. Chicago (IL): American Pain Society, 2004
Cherny NJ, Chang V, Frager G, et al. Opioid pharmacotherapy in the management of cancer pain. Cancer 1995; 76(11): 1283–93
Cherny NI, Portenoy RK. The management of cancer pain. CA Cancer J Clin 1994; 44(2): 262–303
Levy MH. Pharmacologic treatment of cancer pain. N Engl J Med 1996; 335(15): 1124–32
Lawlor P, Turner K, Hanson J, et al. Dose ratio between morphine and hydromorhone in patients with cancer pain: a retrospective study. Pain 1997; 72(1-2): 79–85
Lawlor PG, Turner KS, Hanson J, et al. Dose ratio between morphine and methadone in patients with cancer pain. Cancer 1998; 82(6): 1167–73
Muijers RBR, Wagstaff AJ. Transdermal fentanyl: an updated review of its pharmacological properties and therapeutic efficacy in chronic cancer pain control. Drugs 2001; 61(15): 2289–307
Janssen Pharmaceutica. Duragesic (fentanyl transdermal system) full prescribing information. Titusville (NJ): Janssen Pharmaceutica, 2001
Kornick CA, Santiago-Palma J, Moryl N, et al. Benefit-risk assessment of transdermal fentanyl for the treatment of chronic pain. Drug Saf 2003; 26(13): 951–73
Donner B, Zenz M, Tryba M, et al. Direct conversion from oral morphine to transdermal fentanyl: a multicenter study in patients with cancer pain. Pain 1996; 64(3): 527–34
Breitbart W, Chandler S, Eagel H, et al. An alternative algorithm for dosing of transdermal fentanyl for cancer-related pain. Oncology (Huntingt) 2000 May; 14(5): 695–705
Higgs C, Vella-Brincat J. Withdrawal with transdermal fentanyl. J Pain Symptom Manage 1995; 10: 4–5
Zenz M, Donner B, Strumpt M. Withdrawal symptoms during therapy with transdermal fentanyl (fentanyl TTS). J Pain Symptom Manage 1994; 9(1): 54–5
Radbruch L, Eisner F. Clinical experience with transdermal fentanyl for the treatment of cancer pain in Germany. Keio J Med 2004; 53(1): 23–9
Zech D, Grond S, Lynch J, et al. Transdermal fentanyl and initial dose finding with patient controlled analgesia in cancer pain: a pilot study with 20 terminally ill cancer patients. Pain 1992; 50(3): 293–301
Kornick CA, Santiago-Palma J, Schulman G, et al. A safe and effective method for converting patients from transdermal to intravenous fentanyl for the treatment of acute cancer-related pain. Cancer 2003; 97: 3121–4
Kornick CA, Santiago-Palma J, Khojainova N, et al. A safe and effective method for converting patients from intravenous to transdermal fentanyl. Cancer 2001; 92: 3056–61
Cleary JF. Pharmacokinetic and pharmacodynamic issues in the treatment of breakthrough pain. Semin Oncol 1997; 24 (5 Suppl. 16): 13–9
Payne R, Chandler S, Einhaus M. Guidelines for the clinical use of transdermal fentanyl. Anti Cancer Drugs 1995; 6(3): 50–3
The TTS Fentanyl Multicentre Study Group. Transdermal fentanyl in cancer pain. J Drug Dev 1994; 6(3): 93–7
Southam MA. Transdermal fentanyl therapy: system design, pharmacokinetics and efficacy. Anticancer Drugs 1995; 6 Suppl. 3: 29–34
Portenoy RK, Southam M, Gupta SK. Transdermal fentanyl for cancer pain: repeated dose pharmacokinetics and efficacy. Anesthesiology 1993; 78(1): 36–43
Ahmedzai S, Brooks D. Transdermal fentanyl versus sustainedrelease oral morphine in cancer pain: preference, efficacy, and quality of life. TTS-Fentanyl Comparative Trial Group. J Pain Symptom Manag 1996; 13(5): 254–61
Allan L, Hays H, Jensen NH, et al. Randomised crossover trial of transdermal fentanyl and sustained release oral morphine for treating chronic non-cancer pain. BMJ 2001; 322(7295): 1154–8
Wong JO, Chiu GL, Tsaio CJ, et al. Comparison of oral controlled-release morphine with transdermal fentanyl in terminal cancer pain. Acta Anaesthesiol Sin 1997; 35(1): 25–32
Kongsgaard UE, Poulain P. Transdermal fentanyl for pain control in adults with chronic cancer pain. Eur J Pain 1998; 2(1): 53–62
Evans HC, Easthope SE. Transdermal buprenorphine. Drugs 2003; 63(19): 1999–2010
Bohme K. Burprenorphine in a transdermal therapeutic system: a new option. Clin Rheumatol 2002; 21 Suppl. 1: S13–116
Budd K. Buprenorphine and the transdermal system: the ideal match in pain management. Int J Clin Pract Suppl 2003; 133: 9–14
Radbruch L, Vielvoye-Kerkmeer A. Buprenorphine TDS: the clinical development, rationale and results. Int J Clin Pract Suppl 2003; 133: 15–8
Radbruch L. Buprenorphine TDS: use in daily practice, benefits for patients. Int J Clin Pract Suppl 2003; 133: 19–24
Sittl R, Griessinger N, Likar R. Analgesic efficacy and tolerability of transdermal buprenorphine in patients with inadequately controlled chronic pain related to cancer and other disorders: a multicenter, randomized, double-blind, placebo-controlled trial. Clin Ther 2003; 25(1): 150–68
Bohme K, Kikar R. Efficacy and tolerability of a new opioid analgesic formulation, buprenorphine transdermal therapeutic system (TDS), in the treatment of patients with chronic pain: a randomized, double-blind, placebo-controlled study. Pain Clinic 2003; 15(2): 193–202
Acknowledgements
This article was supported by the Pharmacoeconomics & Pharmacoepidemiology Research Unit, Washington State University, Pullman, Washington, USA. The author reports no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Skaer, T.L. Practice Guidelines for Transdermal Opioids in Malignant Pain. Drugs 64, 2629–2638 (2004). https://doi.org/10.2165/00003495-200464230-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200464230-00002