Abstract
Purpose
Perineural catheter insertion using ultrasound guidance alone is a relatively new approach. Previous studies have shown that ultrasound-guided catheters take less time to place with high placement success rates, but the analgesic efficacy compared with the established stimulating catheter technique remains unknown. We tested the hypothesis that popliteal-sciatic perineural catheter insertion relying exclusively on ultrasound guidance results in superior postoperative analgesia compared with stimulating catheters.
Methods
Preoperatively, subjects receiving a popliteal-sciatic perineural catheter for foot or ankle surgery were assigned randomly to either ultrasound guidance (bolus via needle with non-stimulating catheter insertion) or electrical stimulation (bolus via catheter). We used 1.5% mepivacaine 40 mL for the primary surgical nerve block and 0.2% ropivacaine (basal 8 mL·hr−1; bolus 4 mL; 30 min lockout) was infused postoperatively. The primary outcome was average surgical pain on postoperative day one.
Results
Forty of the 80 subjects enrolled were randomized to each treatment group. One of 40 subjects (2.5%) in the ultrasound group failed catheter placement per protocol vs nine of 40 (22.5%) in the stimulating catheter group (P = 0.014). The difference in procedural duration (mean [95% confidence interval (CI)]) was −6.48 (−9.90 - −3.05) min, with ultrasound requiring 7.0 (4.0-14.1) min vs stimulation requiring 11.0 (5.0-30.0) min (P < 0.001). The average pain scores of subjects who provided data on postoperative day one were somewhat higher for the 33 ultrasound subjects than for the 26 stimulation subjects (5.0 [1.0-7.8] vs 3.0 [0.0-6.5], respectively; P = 0.032), a difference (mean [95%CI]) of 1.37 (0.03-2.71).
Conclusion
For popliteal-sciatic perineural catheters, ultrasound guidance takes less time and results in fewer placement failures compared with stimulating catheters. However, analgesia may be mildly improved with successfully placed stimulating catheters. Clinical trial registration number NCT00876681.
Résumé
Objectif
L’insertion de cathéters périnerveux à l’aide d’échoguidage seul est une approche relativement récente. Des études antérieures ont montré que les cathéters échoguidés requièrent moins de temps de positionnement et offrent un taux élevé de réussite du positionnement, mais nous ne savons pas comment leur efficacité analgésique se compare à celle d’une technique établie de cathéter stimulant. Nous avons testé l’hypothèse selon laquelle un cathéter périnerveux sciatique poplité inséré uniquement à l’aide d’échoguidage donne une meilleure analgésie postopératoire que les cathéters stimulants.
Méthode
Les patients chez lesquels on allait installer un cathéter périnerveux sciatique poplité pour une chirurgie du pied ou de la cheville ont été randomisés avant l’opération à une technique d’échoguidage (bolus via l’aiguille avec l’insertion d’un cathéter non stimulant) ou à la stimulation électrique (bolus via un cathéter). Nous avons utilisé 40 mL de mépivacaïne 1,5 % pour le bloc nerveux chirurgical primaire et de la ropivacaïne 0,2 % (analgésie de base 8 mL·h−1; bolus 4 mL; verrouillage de 30 min) a été perfusée après l’opération. La douleur chirurgicale moyenne au jour postopératoire un était le critère d’évaluation principal.
Résultats
Quarante des 80 patients inscrits à l’étude ont été randomisés dans chaque groupe de traitement. Le positionnement du cathéter selon le protocole a échoué chez un patient sur 40 (2,5 %) dans le groupe échoguidé comparativement à 9 sur 40 (22,5 %) dans le groupe cathéter stimulant (P = 0,014). La différence de durée de l’intervention (moyenne [intervalle de confiance (IC) 95 %]) était de –6,48 (−9,90 - −3,05), l’échoguidage nécessitant 7,0 (4,0-14,1) min contre 11,0 (5,0-30,0) min pour la technique de stimulation (P < 0,001). Les scores de douleur moyens des patients ayant fourni des données au jour postopératoire un étaient un peu plus élevés pour les 33 patients du groupe échoguidage comparativement aux 26 patients du groupe stimulation (5,0 [1,0-7,8] vs. 3,0 [0,0-6,5], respectivement; P = 0,032), soit une différence (moyenne [IC 95 %]) de 1,37 (0,03-2,71).
Conclusion
Lorsque cette technique est utilisée avec des cathéters périnerveux sciatiques poplités, l’échoguidage requiert moins de temps et entraîne moins d’échecs de positionnement que les cathéters stimulants. Toutefois, l’analgésie pourrait être légèrement meilleure avec un cathéter stimulant bien placé. Numéro d’enregistrement de l’étude clinique: NCT00876681.
Similar content being viewed by others
Continuous popliteal-sciatic nerve blocks have demonstrated efficacy in reducing pain, decreasing supplemental opioid requirements and side effects, and improving sleep quality for patients undergoing foot and/or ankle surgery.1,2 There is evidence suggesting that the insertion of stimulating catheters may have benefits over non-stimulating catheters, e.g., faster surgical block onset,3 improved analgesia,4 decreased supplemental opioid requirements,4-6 and a reduced consumption of local anesthetic during patient-controlled perineural analgesia5 (presumably by placing the catheter tip closer to the target nerves). However, in all of these studies, the non-stimulating catheters were placed blindly through an insulated needle after initially using electrical current to localize the target peripheral nerve(s).
More recently, placement of perineural catheters using ultrasound guidance alone has been described.7,8 In one particular ultrasound-guided technique, local anesthetic solution is injected via the placement needle following non-stimulating catheter insertion, resulting in a 50% reduction in catheter placement time compared with a traditional stimulating catheter technique and without a decrease in insertion success rate.9 However, to what extent the postoperative analgesia produced by this new ultrasound-guided technique compares with well-established stimulating catheter techniques remains unknown. Therefore, we tested the hypothesis that this ultrasound-guided technique for placing popliteal-sciatic perineural catheters results in superior postoperative analgesia compared with stimulating catheters placed using electrical current alone.
Methods
The Institutional Review Board (University of California, San Diego School of Medicine, San Diego, CA, USA) approved the protocol and oversaw the study through data analysis. The trial was prospectively registered at clinicaltrials.gov (NCT00876681). Patients offered enrolment included adults (≥ 18 yr) who were scheduled for at least moderately painful orthopedic surgery of the foot and/or ankle and who desired and were approved for a continuous popliteal-sciatic nerve block for postoperative analgesia. Exclusion criteria included known neuropathy of any etiology in the surgical extremity, pregnancy, incarceration, current chronic opioid use (daily opioid consumption of > 10 mg oxycodone equivalent for more than the previous four weeks), history of alcohol or opioid abuse, and inability to communicate with the investigators and hospital staff.
Following written informed consent, the subjects were randomized to one of two treatment groups, i.e., electrical stimulation (ES) with a stimulating catheter or ultrasound (US) guidance with a non-stimulating catheter, using a computer-generated randomization table based in a secure password-protected encrypted central server (www.PAINfRE.com, General Clinical Research Center, Gainesville, FL, USA). Randomization was implemented by the UCSD Investigational Drug Service in blocks of 40 subjects, ensuring balanced numbers between groups. All catheter insertion procedures were performed either by an attending physician with extensive experience with both placement techniques or by a regional anesthesia trainee directly supervised one-on-one by the attending physician.
A peripheral intravenous catheter was inserted in all subjects, standard non-invasive monitors were applied, supplemental oxygen was administered via a face mask, and the subjects were placed in the prone position. Midazolam and fentanyl intravenous were titrated for patient comfort, while ensuring that patients remained responsive to verbal cues. If necessary, the area that would subsequently be covered by the catheter dressing was shaved. Landmarks were drawn for all subjects; the area was cleansed with chlorhexidine gluconate and isopropyl alcohol (ChloraPrep One-Step, Medi-Flex Hospital Products, Inc., Overland Park, KS, USA), and a clear sterile fenestrated drape was applied. The nerve stimulator (ES group) or ultrasound (US group) were readied for use.
Electrical stimulation technique
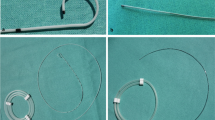
Using a previously described method that was slightly modified, subjects randomized to the ES technique had the sciatic nerve located with a nerve stimulator attached to an insulated needle (Fig. 1).10 A local anesthetic skin wheal was raised 1 cm directly caudad to the apex of the popliteal fossa (bounded by the semi-membranosus muscle medially and the biceps femoris muscle laterally) but not more than 10 cm cephalad to the popliteal fossa skin crease. An 8.9 cm 17 G insulated needle (StimuCath, Arrow International, Reading, PA, USA) was inserted through the skin wheal with the long axis of the needle initially at a 45º angle to the skin/gurney and with the bevel directed cephalad. The needle was connected to a nerve stimulator (Stimuplex-DIG, B. Braun Medical, Bethlehem, PA, USA) set initially at 1.2 mA, 2 Hz, and impulse duration of 0.1 msec. If the sciatic nerve was not identified after 5-8 cm of insertion (depending on patient body habitus), the needle was redirected systematically until foot/toe plantar flexion could be evoked and maintained with a current amplitude from 0.30 to 0.60 mA.
Illustration of a perineural catheter insertion technique employing electrical stimulation alone via a stimulating catheter; Panel 1: sciatic nerve and distal branches (tibial and common peroneal nerves) in the popliteal fossa; Panel 2: following nerve localization, the placement needle angle is lowered prior to catheter insertion; Panel 3: a stimulating catheter is deployed past the placement needle tip while maintaining the desired evoked motor response; Panel 4: the local anesthetic bolus is administered via the catheter
The 19 G catheter was then placed through the length of the needle, and the nerve stimulator connecting wire was transferred from the needle to the catheter, which has a conducting wire through its length in order to deliver current to its tip (Fig. 1, first Panel). The stimulating current was allowed to be increased up to 0.80 mA, and the catheter was advanced 5 cm beyond the needle tip. If plantar flexion decreased as the stimulating catheter was advanced, the catheter was withdrawn into the needle, the needle was redirected or rotated, and the catheter was re-advanced.
Once a catheter was successfully advanced 5 cm further than the needle tip, the needle itself was withdrawn over the catheter and the catheter stylet was removed (Fig. 1, second and third Panels). The catheter was tunnelled subcutaneously 5-7 cm in a lateral direction using the included needle stylet and a 17 G insulated needle. The injection port was attached to the end of the catheter; the nerve stimulator was attached to the injection port, and the minimum current resulting in muscle contraction was noted. The catheter was secured with sterile liquid adhesive, an occlusive dressing, and an anchoring device (StatLock, Venetec International, San Diego, CA, USA) to affix the catheter hub to the patient. Following negative aspiration, 40 mL of anesthetic solution was injected via the catheter with gentle aspiration between divided doses. The injectate contained 1.5% mepivacaine and epinephrine 2.5-5.0 μg·mL−1.
Ultrasound technique
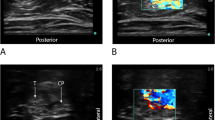
Subjects randomized to the US technique had their target nerve located using ultrasound guidance alone (Fig. 2). With a high-frequency linear array transducer (HFL38, SonoSite M-Turbo, Bothell, WA, USA) in a sterile sleeve, the sciatic nerve was identified in short-axis at the apex of the popliteal fossa. Once the optimal image of the sciatic nerve was obtained, a local anesthetic skin wheal was raised lateral to the US transducer. An 8.9 cm 17 G Tuohy-tip needle (FlexTip, Arrow International, Reading, PA, USA) was inserted through the skin wheal in-plane beneath the US transducer and directed medially toward the sciatic nerve with the bevel directed posteriorly as described previously (Fig. 2, first Panel).9 Local anesthetic solution (1.5% mepivacaine 40 mL with epinephrine 2.5-5.0 μg·mL−1) was injected in divided doses circumferentially around the target nerve via the needle (Fig. 2, second Panel).
Illustration of a perineural catheter insertion technique employing ultrasound guidance alone with a non-stimulating catheter; Panel 1: the placement needle is directed in-plane toward the target nerve; Panel 2: the local anesthetic bolus is administered via the needle; Panel 3: a non-stimulating catheter may bypass the target nerve after insertion and require withdrawal; Panel 4: the non-stimulating catheter in proper position in proximity to the target nerve
A 19 G catheter (FlexTip, Arrow International, Reading, PA, USA) was then placed through the length of the needle and advanced 5 cm beyond the needle tip (Fig. 2, third and fourth Panels). Once a catheter had been inserted, the needle itself was withdrawn over the catheter. The injection port was attached to the end of the catheter, and the catheter tip position was inferred by injecting 1 mL of air via the catheter under US, slightly withdrawn if necessary, and another 1 mL of air injected to confirm accurate catheter tip placement.11,12 The catheter was not tunnelled further but was dressed and secured in a similar manner to the ES technique.
If a catheter could not be placed per protocol within 30 min, the placement was considered a failure, and the time was recorded as 30 min. In such cases, the attending physician had the option of attempting catheter placement using the alternate method. Subjects having a failed catheter placement were removed from further study.
Fifteen minutes post-injection, block onset was evaluated and scored in the affirmative if patients were unable to plantar flex the ankle and experienced decreased sensory perception to light touch on the plantar surface of the foot compared with the contralateral limb. Subjects with a successful surgical block were retained in the study. If the duration of the initial surgical block required extension, a secondary anesthetic bolus of 1.5% mepivacaine 20 mL with epinephrine 2.5-5.0 μg·mL−1 was administered via the catheter following negative aspiration. Postoperatively, each perineural catheter was attached to an electronic portable infusion pump (Pain Pump 2 BlockAid, Stryker Instruments, Kalamazoo, MI, USA) set to deliver 0.2% ropivacaine (basal rate of 8 mL·hr−1; patient-controlled bolus of 4 mL; 30 min lockout interval). Subjects were prescribed an oral opioid (oxycodone 5 mg tablets) for breakthrough postoperative pain inadequately treated by the perineural ropivacaine infusion/bolus.
For this study, the primary outcome was the average pain score on postoperative day one (POD 1), approximately 24 hr following surgery. Subjects reported their pain scores using a numeric rating scale (NRS) of 0-10 (0 = no discomfort; 10 = the worst discomfort imaginable). With regard to secondary outcome measurements, the time (min) to perform the procedure was recorded from the instant when the ultrasound transducer (US group) or catheter placement needle (ES group) first touched the patient to the instant when the catheter placement needle was removed following catheter placement (both groups). Time for catheter tunnelling (ES group) was not included in this measurement. The occurrence of inadvertent vascular puncture during catheter placement was noted, if it happened. Immediately following catheter placement, subjects were asked to rate their procedure-related discomfort on the same numeric rating scale. Total perioperative fentanyl (μg) administered for catheter placement and during surgery was recorded. On POD 1, 24 hr following surgery, subjects were also asked to rate their worst pain scores (NRS) in the preceding period as well as oral oxycodone consumption (mg) and occurrence of catheter leakage.
Statistical analysis
We considered a difference of 1.25 on the NRS to be clinically relevant for moderately to severely painful orthopedic surgery. Based on this expected difference, a standard deviation in each group of two and assuming a two-sided type I error protection of 0.05 and a power of 0.80, approximately 40 patients were required in each group (StatMate 2.0, GraphPad Software, San Diego, CA, USA).
For normally distributed data, comparisons of independent samples were performed using Student’s t test. For continuous data in distributions other than normal, the Mann-Whitney U test was used. The Z-test or a Fisher’s exact test (expected cell size < 5 in any category) was used for comparisons of categorical variables. A two-sided P < 0.05 was considered statistically significant for the primary outcome. Statistically significant findings in secondary outcomes should be interpreted as suggestive, requiring confirmation in a prospective trial before being considered definitive.13
Results
Eighty subjects were enrolled and assigned randomly to treatment groups during the period from May 2008 until April 2009. Demographic, anthropometric, and surgical characteristics were similar between groups (Tables 1 and 2). One (2.5%) of the subjects randomized to US (n = 40), failed catheter placement, and one additional subject failed to develop a successful nerve block. Nine (22.5%) of the subjects randomized to ES (n = 40) failed catheter placement per the ES protocol (P = 0.014 compared with the US group), and four subjects had catheters placed according to protocol but did not develop a surgical block. All failed catheters were subsequently replaced using the US protocol, even though these subjects were withdrawn from postoperative data collection. Five of the nine ES catheter placement failures resulted from an inability to elicit a motor response via the insulated needle at a current below 0.6 mA. Four failures resulted from an inability to maintain the proper motor response via the stimulating catheter. The failures occurred with a current below 0.8 mA as the catheter was advanced past the needle tip, as specified in the protocol. There were two protocol deviations. In the US group, two subjects received a 0.5% ropivacaine bolus via the catheter following surgery, and one subject’s infusion device stopped working during the first night after surgery and was replaced the following day.
Regarding the primary outcome, postoperative pain scores were not collected from seven subjects in the US group and 14 subjects in the ES group due to study withdrawal or loss to follow up (P = 0.075). From the data collected, the average NRS pain scores (median [10th-90th percentiles]) were lower in subjects with a stimulating catheter the day following surgery than in subjects who received an ultrasound-guided non-stimulating catheter (3.0 [0.0-6.5] vs 5.0 [1.0-7.8], respectively; P = 0.032) (Fig. 3). This difference (mean [95% CI]) was 1.37 (0.03-2.71) in favour of the ES group.
Regarding secondary outcomes, worst pain scores on POD 1 were 6.5 (3.0-10.0) for ES vs 8.0 (2.2-10.0) for US (P = 0.312) and with a difference (mean [95%CI]) of 0.64 (−0.82-2.09). Non-stimulating perineural catheters placed by US took 7.0 (4.0-14.1) min vs 11.0 (5.0-30.0) min for stimulating catheters placed with ES (P < 0.001) with a difference (mean [95%CI]) of -6.48 (−9.90 - −3.05) in favour of US. There were no vascular punctures using ultrasound guidance vs five in the stimulation group (P = 0.021). There were no statistically significant differences in other secondary outcomes (Tables 3 and 4).
Discussion
Many acceptable approaches have been described for popliteal-sciatic perineural catheter insertion. The results of this randomized clinical trial provide preliminary evidence that an established stimulating catheter technique may provide slightly better postoperative analgesia compared with a newer ultrasound-guided non-stimulating catheter technique. However, these results should be interpreted as suggestive and not definitive given the number of subjects withdrawn and lost to follow up. The newer ultrasound-guided technique does allow for insertion of a perineural catheter in less time and with fewer catheter placement failures than the stimulating catheter technique. In theory, the improved analgesia from stimulating catheters results from catheter tip placement closer to the target nerve and effectively “testing” the catheter by administering the local anesthetic bolus via the catheter. Unfortunately, the stimulating catheter technique is more difficult to perform—resulting in more insertion failures and a longer average procedural duration. Based on our comparison of these two techniques, practitioners may choose faster and more successful catheter insertion or a mild improvement in postoperative analgesia. The difference in average pain scores of 5 (US) vs 3 (ES) can be considered clinically significant, since pain scores > 4 are routinely treated with breakthrough opioid analgesic medications.14
This investigation was a comparative efficacy study that provides relevant information to practitioners on optimizing patient care by directly comparing two current treatments. Our goal is not to compare “ultrasound” to “nerve stimulation” in general, but rather to provide practitioners with relevant clinical information directly comparing two currently utilized perineural catheter insertion techniques that differ from each other in many ways. Both techniques have been amply reported in the literature.8,10,11,15–18
In comparative efficacy research involving regional anesthesia and analgesia, the use of different catheter designs for each treatment group is well-established. When comparing the clinical efficacy of lumbar epidural with femoral perineural local anesthetic infusion,19,20 different catheter designs are utilized to optimize the effect of each of the two different approaches. Previous randomized controlled studies have evaluated the duration of time for catheter placement using the ultrasound-guided catheter insertion technique9,15,21,22; but the relative analgesia this technique provides compared with a more traditional nerve stimulation technique using a stimulating catheter has remained unknown to date. We cannot speculate to what extent alternative ultrasound-guided perineural catheter insertion techniques will compare with other ultrasound- and stimulating-guided catheter approaches or even combined ultrasound-stimulation techniques, and it is clear that further research involving randomized clinical trials is required to optimize perineural catheter placement methods.
The median time difference of four minutes gained by US over ES is similar to the results of a previous study9 and may be clinically significant in practice environments with high surgical volume and rapid turnover. The US technique offers further time savings as a result of: 1) avoiding subcutaneous tunnelling and injection of local anesthetic bolus via the catheter; and 2) injecting the local anesthetic bolus in the US-guided technique via the needle prior to catheter placement to speed nerve block onset and potentially reduce anesthesia-controlled time.23
The failure to place nine (22.5%) of the 40 stimulating catheters in the ES group deserves comment. By protocol, “failure” occurred when a motor response could not be evoked within 30 min, the electrical current via the needle could not be reduced below 0.6 mA with an evoked motor response, or the stimulating current via the catheter could not be reduced below 0.8 mA while retaining an evoked motor response with the catheter inserted 5 cm beyond the needle tip. Placement needles and stimulating catheters may have been positioned in proximity to the target nerve despite failure to evoke a motor response at the threshold current criteria.24 These criteria may be unnecessarily restrictive6 and likely may have affected the ES group success rate. However, strict catheter placement criteria provides definitive benefits in clinical research,4,5 and the protocol used in the current study is similar to one used previously.10,16 The 30 min time limit in the current study, while generous in clinical practice, may explain the difference in success rates compared with previous investigations without time limits but with nearly identical catheter placement protocols.25,26 Further, nearly all procedures for the present study were performed by trainees, while previous investigations relying exclusively on experienced attending physicians have reported much higher success rates.10,16,27 Additionally, differences in patient populations and sampling may have influenced the success rate.
We acknowledge the limitations of this study. The number of subjects withdrawn from postoperative data collection or lost to follow up introduces selection bias into the analysis of the primary outcome. We can only speculate why more subjects in the ultrasound group were unavailable by phone the day following surgery. If subjects with minimal pain elected not to answer the phone while those with severe pain were more inclined to speak to clinicians, the sample would be biased in favour of higher postoperative pain scores. Therefore, the results of the present study should be interpreted as suggestive and not conclusive until they can be validated further by subsequent randomized clinical trials.
The lack of masking, a major limitation of this investigation, was accepted, as it was deemed impossible to mask investigators or patients to treatment group. In addition, the two methods for perineural catheter placement that were compared in this study employed different equipment and techniques, as discussed previously. The results of the present study apply specifically to the two techniques and the associated equipment under investigation. These results should not be extrapolated to all electrical stimulation and ultrasound-guided techniques.
Another important limitation affecting the primary outcome is the heterogeneous etiology of postoperative pain. Subjects’ pain experience is invariably influenced by factors other than the method of perineural catheter insertion (e.g., site of surgery, surgical technique, tourniquet time, and nerve distributions not covered by the primary nerve block catheter). While randomization is intended to distribute these unexpected factors equally between study groups, they still may exert unintended influence on the primary outcome measurement.
Our results may not apply to all practices since trainees placed nearly all of the perineural catheters included in this study. This factor may have affected the time measurements and may not be representative of actual procedure times in other practice environments. Lastly, the results of this study apply only to popliteal-sciatic perineural catheters and should not be inferred to other insertion sites, as perineural anatomy directly affects catheter insertion and infusion characteristics.16,25,28
In summary, this randomized comparative efficacy study demonstrates that a stimulating catheter, i.e., the popliteal-sciatic perineural catheter, may mildly improve analgesia on the first postoperative day compared with a technique relying exclusively on ultrasound guidance to place a non-stimulating catheter. However, the ultrasound-guided technique requires less time to perform and results in fewer placement failures. These results should not be extrapolated to all stimulation or ultrasound-guided techniques or other catheter insertion sites.
References
White PF, Issioui T, Skrivanek GD, Early JS, Wakefield C. The use of a continuous popliteal sciatic nerve block after surgery involving the foot and ankle: does it improve the quality of recovery? Anesth Analg 2003; 97: 1303-9.
Ilfeld BM, Morey TE, Wang RD, Enneking FK. Continuous popliteal sciatic nerve block for postoperative pain control at home: a randomized, double-blinded, placebo-controlled study. Anesthesiology 2002; 97: 959-65.
Stevens MF, Werdehausen R, Golla E, et al. Does interscalene catheter placement with stimulating catheters improve postoperative pain or functional outcome after shoulder surgery? A prospective, randomized and double-blinded trial. Anesth Analg 2007; 104: 442-7.
Rodriguez J, Taboada M, Carceller J, Lagunilla J, Barcena M, Alvarez J. Stimulating popliteal catheters for postoperative analgesia after hallux valgus repair. Anesth Analg 2006; 102: 258-62.
Casati A, Fanelli G, Koscielniak-Nielsen Z, et al. Using stimulating catheters for continuous sciatic nerve block shortens onset time of surgical block and minimizes postoperative consumption of pain medication after halux valgus repair as compared with conventional nonstimulating catheters. Anesth Analg 2005; 101: 1192-7.
Morin AM, Eberhart LH, Behnke HK, et al. Does femoral nerve catheter placement with stimulating catheters improve effective placement? A randomized, controlled, and observer-blinded trial. Anesth Analg 2005; 100: 1503-10.
Swenson JD, Bay N, Loose E, et al. Outpatient management of continuous peripheral nerve catheters placed using ultrasound guidance: an experience in 620 patients. Anesth Analg 2006; 103: 1436-43.
Sandhu NS, Manne JS, Medabalmi PK, Capan LM. Sonographically guided infraclavicular brachial plexus block in adults: a retrospective analysis of 1146 cases. J Ultrasound Med 2006; 25: 1555-61.
Mariano ER, Cheng GS, Choy LP, et al. Electrical stimulation versus ultrasound guidance for popliteal-sciatic perineural catheter insertion: a randomized controlled trial. Reg Anesth Pain Med 2009; 34: 480-5.
Ilfeld BM, Thannikary LJ, Morey TE, Vander Griend RA, Enneking FK. Popliteal sciatic perineural local anesthetic infusion: a comparison of three dosing regimens for postoperative analgesia. Anesthesiology 2004; 101: 970-7.
Sandhu NS, Capan LM. Ultrasound-guided infraclavicular brachial plexus block. Br J Anaesth 2002; 89: 254-9.
Swenson JD, Davis JJ, DeCou JA. A novel approach for assessing catheter position after ultrasound-guided placement of continuous interscalene block. Anesth Analg 2008; 106: 1015-6.
Mariano ER, Ilfeld BM, Neal JM. “Going fishing”-the practice of reporting secondary outcomes as separate studies. Reg Anesth Pain Med 2007; 32: 183-5.
Frasco PE, Sprung J, Trentman TL. The impact of the joint commission for accreditation of healthcare organizations pain initiative on perioperative opiate consumption and recovery room length of stay. Anesth Analg 2005; 100: 162-8.
Mariano ER, Loland VJ, Bellars RH, et al. Ultrasound guidance versus electrical stimulation for infraclavicular brachial plexus perineural catheter insertion. J Ultrasound Med 2009; 28: 1211-8.
Ilfeld BM, Loland VJ, Gerancher JC, et al. The effects of varying local anesthetic concentration and volume on continuous popliteal sciatic nerve blocks: a dual-center, randomized, controlled study. Anesth Analg 2008; 107: 701-7.
Ilfeld BM, Morey TE, Enneking FK. Infraclavicular perineural local anesthetic infusion: a comparison of three dosing regimens for postoperative analgesia. Anesthesiology 2004; 100: 395-402.
Salinas FV, Neal JM, Sueda LA, Kopacz DJ, Liu SS. Prospective comparison of continuous femoral nerve block with nonstimulating catheter placement versus stimulating catheter-guided perineural placement in volunteers. Reg Anesth Pain Med 2004; 29: 212-20.
Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, d’Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology 1999; 91: 8-15.
Singelyn FJ, Deyaert M, Joris D, Pendeville E, Gouverneur JM. Effects of intravenous patient-controlled analgesia with morphine, continuous epidural analgesia, and continuous three-in-one block on postoperative pain and knee rehabilitation after unilateral total knee arthroplasty. Anesth Analg 1998; 87: 88-92.
Mariano ER, Loland VJ, Sandhu NS, et al. Ultrasound guidance versus electrical stimulation for femoral perineural catheter insertion. J Ultrasound Med 2009; 28: 1453-60.
Mariano ER, Loland VJ, Sandhu NS, et al. A trainee-based randomized comparison of stimulating interscalene perineural catheters with a new technique using ultrasound guidance alone. J Ultrasound Med 2010; 29: 329-36.
Marhofer P, Schrogendorfer K, Koinig H, Kapral S, Weinstabl C, Mayer N. Ultrasonographic guidance improves sensory block and onset time of three-in-one blocks. Anesth Analg 1997; 85: 854-7.
Tsai TP, Vuckovic I, Dilberovic F, et al. Intensity of the stimulating current may not be a reliable indicator of intraneural needle placement. Reg Anesth Pain Med 2008; 33: 207-10.
Le LT, Loland VJ, Mariano ER, et al. Effects of local anesthetic concentration and dose on continuous interscalene nerve blocks: a dual-center, randomized, observer-masked, controlled study. Reg Anesth Pain Med 2008; 33: 518-25.
Ilfeld BM, Vandenborne K, Duncan PW, et al. Ambulatory continuous interscalene nerve blocks decrease the time to discharge readiness after total shoulder arthroplasty: a randomized, triple-masked, placebo-controlled study. Anesthesiology 2006; 105: 999-1007.
Fredrickson MJ, Ball CM, Dalgleish AJ, Stewart AW, Short TG. A prospective randomized comparison of ultrasound and neurostimulation as needle end points for interscalene catheter placement. Anesth Analg 2009; 108: 1695-700.
Ilfeld BM, Le LT, Ramjohn J, et al. The effects of local anesthetic concentration and dose on continuous infraclavicular nerve blocks: a multicenter, randomized, observer-masked, controlled study. Anesth Analg 2009; 108: 345-50.
Acknowledgements
The authors gratefully acknowledge the invaluable assistance of the entire operating and recovery room staff at the University of California San Diego Hillcrest (San Diego, CA, USA) and Thornton hospitals (La Jolla, CA, USA). We also acknowledge with thanks David Cheney, Medical Illustrator, from the Mayo Clinic.
Financial Support
Funding for this project provided by NIH grant GM077026 (P.I.: Dr. Ilfeld) from the National Institute of General Medical Sciences (Bethesda, MD, USA); and the Department of Anesthesiology, University of California, San Diego Medical Center (San Diego, CA, USA). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of these entities.
Conflict of interest
Drs. Mariano, Loland, and Ilfeld as well as Ms. Ferguson have received funding for other research investigations from Arrow International (Reading, Pennsylvania, USA) and Stryker Instruments (Kalamazoo, Michigan, USA). These companies had absolutely no input into any aspect of the present study conceptualization, design, and implementation; data collection, analysis and interpretation; or manuscript preparation. Drs. Mariano and Loland conduct continuous peripheral nerve block workshops for Stryker Instruments (Kalamazoo, Michigan, USA). None of the other authors has any personal financial interest in this research.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Mariano, E.R., Loland, V.J., Sandhu, N.S. et al. Comparative efficacy of ultrasound-guided and stimulating popliteal-sciatic perineural catheters for postoperative analgesia. Can J Anesth/J Can Anesth 57, 919–926 (2010). https://doi.org/10.1007/s12630-010-9364-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-010-9364-7