Abstract
Background
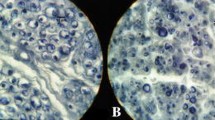
Sciatic nerve is the largest peripheral nerve of the human body. It gives motor and sensitive innervation for the most of lower limb. The aim of the present investigation was revealing his fascicular pattern in relation to microanatomic morphometric characteristics of its connective tissue sheaths.
Methods
The material consisted of sciatic nerve slices, excised from 17 cadavers of humans aging 8–93 years. After routine histologic processing and light microscopic examination of the preparations, morphometric analysis was performed at magnifications of 40 and 630×.
Results
Sciatic nerve showed to be polyfascicular nerve type, with the group pattern of nerve fascicless distribution. The number of fascicless ranged from 27 to 70, whereas the number of fascicless per square millimeter was 1–4. Morphometric and correlation analysis confirmed the significant increase of whole sciatic nerve cross section area, which was associated with the significant increase of its epi- and perineural connective tissue sheaths. Interfascicular sciatic nerve domains of elderly persons contained more adipose tissue. Moreover, already detected loss and degeneration of the large myelinated nerve fibers within fascicles was accompanied by the significant increase of endoneural connective tissue.
Conclusions
In conclusion, our study revealed comparative connective tissue enlargement of human sciatic nerve in the course of aging. These phenomena might influence on result of injured nerve’s surgical reparations. We interpret this finding as non-specific compensatory phenomenon elicited by loss of thickest myelinated nerve fibers, higher vulnerability of remaining ones, and age-dependent decrease of connective tissue elasticity.



Similar content being viewed by others
References
Asbury AK, Johnson PC (1978) Pathology of peripheral nerve. WB Saunders, London, pp 5–55
Azcoitia I, Leonem E, Magnaghi V et al (2003) Progesterone and its derivates dihydroprogesterone and tetrahydroprogesterone reduce myelin fiber morphological abnormalities and myelin fiber loss in the sciatic nerve of aged rats. Neurobiol Aging 24:853–860
Barkmeier JM, Luschei ES (2000) Quantitative analysis of the anatomy of the epineurium of the canine recurrent laryngeal nerve. J Anat 196:85–101
Bendszuc M, Wessing C, Solymosi L et al (2004) MRI of peripheral nerve degeneration and regeneration: correlations with electrophysiology and histology. Exp Neurol 88:171–177
Ceballos D, Cuadras J, Verdú E et al (1999) Morphometric and ultrastructural changes with ageing in mouse peripheral nerve. J Anat 195:563–576
Dawson B, Trapp RG (2004) Basic and Clinical Biostatistics, 4th edn. McGraw-Hill, New York
Drury RAB, Wallington EA (1980) Carleton’s histological technique, 5th edn. Oxford University Press, New York, pp 143–144
Dubowitz V, Brooke MH (1973) Muscle biopsy: a modern approach. WB Saunders Company, London, pp 27–28
Dyck PJ, Thomas PK, Lambert EH et al (1984) Peripheral neuropathy, 2nd edn. WB Saunders Company, London, pp 39–121
Graif M, Seton A, Nerubai J et al (1991) Sciatic nerve: sonographic evaluation and anatomic-pathologic considerations. Radiology 181:405–408
Ikeda K, Haughtoun VM, Ho KC et al (1996) Correlative MR-anatomic study of median nerve. AJR Am J Roentgenol 167:1233–1236
Jacobs JM, Love S (1985) Qualitative and quantitative morphology of human sural nerve at different ages. Brain 108:897–924
Johansson CS, Stenström M, Hildebrand C (1996) Target influence on aging of myelinated sensory nerve fibers. Neurob Aging 17:61–66
Kališnik M (1985) Temelji stereologije. Stereološka sekcija Zveze društev anatomov Jugoslavije, Ljubljana
Korompilias AV, Payatakes AH, Beris AE et al (2006) Sciatic and peroneal nerve injuries. Microsurgery 26:288–294
Lowry A, Wilcox D, Masson EA et al (1997) Immunohistochemical methods for semiquantitative analysis of collagen content in human peripheral nerve. J Anat 91:367–374
Maravilla KR, Bowen BC (1998) Imaging of the peripheral nervous system: evaluation of peripheral neuropathy and plexopathy. AJNR Am J Neuroradiol 19:1011–1023
Melcangi RC, Magnaghi V, Martini L (2000) Aging in peripheral nerves: regulation of myelin protein genes by steroid hormones. Prog Neurobiol 60:291–308
Phillips J, Smit X, De Zoysa N et al (2004) Peripheral nerves in the rat exhibit localized heterogeneity of tensile properties during limb movement. J Physiol 557(3):879–887
Rempel D, Dahlin L, Lundborg G (1999) Pathophysiology of nerve compression syndromes: response of peripheral nerves to loading. J Bone Joint Surg 81:1600–1610
Schröder JM (1972) Altered ratio between axon diameter and myelin sheath thickness in regenerated nerve fibers. Brain Res 45:49–65
Sunderland S (1964) Nerves and nerve injuires, 2nd edn. Churchill-Livingstone, New York, pp 35–49
Tillett RL, Afoke A, Hall SM et al (2004) Investigating mechanical behaviour at a core-sheath interface in peripheral nerve. J Peripher Nerv Syst 9:255–262
Tohgi H, Tsukagaoshi H, Toyokura M (1977) Quantitative changes with age in normal sural nerves. Acta Neuropathol 38:213–220
Topp KS, Boyd BS (2006) Structure and biomechanics of peripheral nerves: nerve responses to physical therapist practice. Phys Ther 86:92–109
Vivo J, Morales JL, Diz A et al (2004) Intracranial portion of the trochlear nerve and dorsal oblique muscle composition in dog: a structural and ultrastructural study. J Morphol 262:708–713
Verdú E, Ceballos D, Vilches J et al (2000) Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst 5:191–208
Verheijen MHG, Chrast R, Burrola P et al (2003) Local regulation of fat metabolism in peripheral nerves. Genes Dev 17:2450–2464
Weller RO, Cervós-Navarro J (1977) Pathology of peripheral nerves. Butterworths, London, pp 5–67
Williams PL, Warwick R, Dyson M, Bannister LH (1995) Gray’s anatomy. Churchill Livingstone, New York, pp 946–957
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sladjana, U.Z., Ivan, J.D. & Bratislav, S.D. Microanatomical structure of the human sciatic nerve. Surg Radiol Anat 30, 619–626 (2008). https://doi.org/10.1007/s00276-008-0386-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00276-008-0386-6