Article Text
Abstract
Background While popularly consumed for its perceived benefits as a sleeping aid, the impact of cannabis on sleep-wake regulation in clinical studies is inconclusive. The purpose of this study was to determine the relationship between cannabis use and nightly sleep duration in a nationally representative dataset.
Methods A cross-sectional analysis of adults was undertaken using the National Health and Nutrition Examination Survey data from 2005 to 2018. Respondents were dichotomized as recent users or non-users if they had used or not used cannabis in the past 30 days, respectively. The primary outcome was nightly sleep duration, categorized as short (<6 hours), optimal (6–9 hours), and long (>9 hours). Multinomial logistic regression was used to adjust for sociodemographic and health-related covariates, and survey sample weights were used in modeling.
Results From a sample representing approximately 146 million adults in the USA, 14.5% reported recent cannabis use. In an adjusted analysis, recent users were more likely than non-users to report both short sleep (OR 1.34, 95% CI 1.12 to 1.59, p<0.001) and long sleep (OR 1.56, 95% CI 1.25 to 1.96, p<0.001). Heavy users (≥20 of the past 30 days) were even more likely to be at the extremes of nightly sleep duration.
Discussion Recent cannabis use was associated with the extremes of nightly sleep duration in a nationally representative sample of adults, with suggestions of a dose–response relationship. Our findings highlight the need to further characterize the sleep health of regular cannabis users in the population.
- analgesia
- epidemiology
- pharmacology
- treatment outcome
- outcomes
Data availability statement
Data are available in a public, open access repository. Data are available on reasonable request. All data for this study and tutorials to access the data are publicly available at https://wwwn.cdc.gov/nchs/nhanes/.
Statistics from Altmetric.com
Introduction
Cannabis use in North America continues to rise in prevalence. Approximately 45 million adults in the USA reported cannabis use in 2019, double the prevalence from the early 2000s.1 This is, at least in part, related to widespread decriminalization in many states over the past decade, as well as scientific or clinical research that have encouraged a reduction in the perceived risk of cannabis use.2 Ongoing research suggests that cannabinoids may have therapeutic value as well, having shown promise for pain relief3 with ongoing efforts to determine their role in anxiety and sleep disorders.
Despite popular use as sleeping aids,4 there are mixed findings about the effects of cannabis and cannabinoids on various sleep metrics such as duration, architecture, and quality.5 Both sleep-wake regulation and human endocannabinoid physiology are immensely complex, and their interaction is not yet fully understood. While animal and in vitro studies have begun to elucidate these pathways, clinical studies with humans have typically been small and heterogeneous in design, leading to mixed results overall.6–8
At the same time, sleep deprivation and insufficiency in the population has become a major public health concern.9 Experts recommend 7–9 hours of sleep per night, yet only two-thirds meet this guideline and almost half of all American adults report daytime sleepiness nearly every day.10 Chronic sleep disturbances increase overall morbidity and mortality related to traumatic accidents, cardiovascular comorbidities, psychiatric disorders, and chronic pain.11–13 Identifying or developing safe interventions to improve sleep health is thus a priority.
Characterizing relationships between current cannabis use trends and sleep patterns may help guide targeted clinical intervention and policy development to improve health outcomes at the patient and population levels. The purpose of this study was to determine the relationship between recent cannabis use and nightly sleep duration in a nationally representative data set.
Methods
Study population
Data for this study were obtained from the National Health and Nutrition Examination Survey (NHANES), which is a cross-sectional survey designed by the National Center for Health Statistics (NCHS) and Centers for Disease Control and Prevention. The NHANES is designed to yield nationally representative data for the non-institutionalized civilian population in the continental USA. This is achieved using a multistage area probability sample selection (1): selection of primary sampling units (PSUs),(2) segments within PSUs (one or more blocks containing a cluster of households),(3) households within segments, and (4) at least one participant within each household. Sample weights and adjustments are then made to account for oversampling and control for non-response.14 For this present study, a data set was constructed using publicly available files from seven 2-year cycles of NHANES responses (2005–2006, 2007–2008, 2009–2010, 2011–2012, 2013–2014, 2015–2016, and 2017–2018). The study population consisted of all respondents to the NHANES cannabis questionnaires, which were only administered to adults aged 20–59 years.
Exposure
Cannabis use was the primary exposure variable for this study. While there is no single definition for regular cannabis use across the scientific literature,15 we dichotomized participants as: (1) non-users if they reported no cannabis use in the past 30 days or; (2) recent users if they had reported any cannabis use in the past 30 days. This definition has been consistently used with previous studies examining cannabis use in the NHANES data set.16
Outcomes
The primary outcome for this study was nightly sleep duration categorized as short, optimal, or long. Based on previous studies examining sleep metrics and outcomes, we defined short sleep as <6 hours and long sleep as >9 hours on average weeknights or worknights.17 Secondary outcomes included: (1) whether participants reported having any trouble falling asleep, staying asleep, or sleeping too much in the past 2 weeks; (2) whether patients had ever told any physician about having any trouble with sleep (eg, duration, patterns, or habits), which was determined by any score greater than 0 for Item #3 of the Patient Health Questionnaire-9; and (3) whether participants experienced daytime sleepiness often, which was defined as feeling overly sleepy on at least 5 of the last 30 days (only captured in 2005–2006, 2007–2008, 2015–2016, and 2017–2018 cycles).
Covariates
Covariates were selected a priori based on biological plausibility of being a confounder in the relationship between the exposure and primary outcome. Demographic characteristics included age (categorical: 20–29, 30–39, 40–49, or 50–59 years old), sex, and race (categorical: Hispanic, white, black, or other) were included. Education beyond high school and number of hours worked per week (categorical: <20, 20–40, or >40 hours) were considered as socioeconomic factors. Health-related variables such as history of hypertension, diabetes, coronary artery disease, body mass index (categorical: <25 kg/m2, 25–30 kg/m2, or ≥30 kg/m2), smoking, heavy alcohol use (≥4 drinks per day, on average), and prescriptions within the past 30 days for the following classes of medications were all considered binary variables: opioids, benzodiazepines, ‘Z drugs’, barbiturates, other sedatives, and stimulants (see online Supplementary Table A for the full list of agents included in these classes).18 19 Finally, the 2-year survey cycle in which participants were interviewed or examined was included in statistical modeling. For any covariate for which >5% of survey participants were missing data, a separate level was coded for the missing values to retain these large samples in analyses. Respondents with missing values for exposure variables, outcome variables, or any other covariates were removed from analyses.
Supplemental material
Data analysis
Weighted differences in baseline covariates and outcome variables between exposure groups were analyzed using χ2 tests. Multinominal logistic regression was used to determine the association between recent cannabis use and sleep duration categories in the primary analysis while accounting for covariates. A multinominal model was used to allow comparison of the outcomes of interest (‘short’ and ‘long’ sleep) to a reference level of ‘optimal’ sleep. The relationship between recent cannabis use and the binary secondary outcomes was then assessed with adjusted multiple logistic regression models. All regression models took into consideration NHANES sample weights which were adjusted by the number of years of survey data to represent a single year and population. All covariates were included in the model without further selection. To account for potential differences over time related to evolving attitudes towards cannabis use, an interaction term between cannabis use and the year of survey administration was added to the regression model. The regression term was found to be not statistically significant and thus was not included in the primary analysis. No other interaction terms were considered.
Further analyses were subsequently performed to test the robustness of our primary model. First, to test for a dose effect, recent cannabis users were further categorized as moderate or heavy users if they had, respectively, used <20 or ≥20 days of the past 30 days. Second, recent users were compared with former users, who were defined as having a history of use but none in the past 30 days.
Significance was tested through two-tailed tests at a significance level of p<0.05 for all described analyses. All data analyses were performed using R V.3.5.2 (R Core Team, Vienna, Austria). Regression models that accounted for adjusted sample weights and maintained the complex survey design of the NHANES were built using the open source ‘survey’ and ‘svrepmisc’ packages, with guidance from published statistical methods.14 20 The sample size was based on the available data, no further observations were removed, and no a priori power calculations were performed.
Results
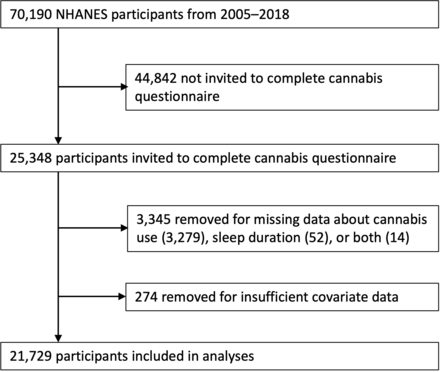
From 2005 to 2018, surveys about cannabis use were administered to a total of 25,348 participants through the NHANES. After excluding participants with missing outcome, exposure, or covariate data (other than the exceptions described above), 21,729 were included in analyses (figure 1), representing an estimated 146,417,873 adults from the USA. A total of 3132 (14.5%) reported cannabis use in the past 30 days. Sample characteristics grouped by cannabis exposure are presented in table 1.
Participant inclusion flow chart from the National Health and Nutrition Examination Survey (NHANES) 2005–2018.
Characteristics of included participants from the National Health and Nutrition Examination Survey, 2005–2018
The mean self-reported sleep duration of all included survey respondents was 6.97±1.47 hours; there was no difference between those with recent cannabis use and those without. Among all survey respondents, 12.2% reported <6 hours, and 3.6% reported >9 hours per night (table 2). Compared with those with no cannabis use in the past 30 days, recent users were more likely to report sleeping <6 hours per night (15.7% vs 11.6%; unadjusted OR 1.47, 95% CI 1.26 to 1.71, p<0.001), or >9 hours per night (6.5% vs 3.1%; unadjusted OR 2.26, 95% CI 1.88 to 2.73, p<0.001). After adjusting for covariates, the association persisted with cannabis users more likely to endorse either short sleep (adjusted OR (aOR) 1.34, 95% CI 1.12 to 1.59, p<0.001) or long sleep (aOR 1.56, 95% CI 1.25 to 1.96, p<0.001) (table 3; full regression model displayed in online supplementary table Bonline Supplementary Table B).
Self-reported sleep outcomes of included participants from the National Health and Nutrition Examination Survey, 2005–2018
Weighted and adjusted ORs for cannabis use and nightly sleep duration
Recent cannabis users were also more likely to report difficulty falling asleep, staying asleep, or sleeping too much in the past 2 weeks (aOR 1.31, 95% CI 1.18 to 1.45, p<0.001), and having ever told a physician about having trouble with sleep (aOR 1.29, 95% CI 1.13 to 1.47, p<0.001) (table 2). Recent cannabis exposure was not associated with frequent daytime sleepiness (aOR 1.00, 95% CI 0.88 to 1.14, p=0.94).
When recent users were further categorized by frequency of use, moderate users (<20 of the past 30 days) were more likely to have long sleep (aOR 1.47, 95% CI 1.13 to 1.91, p<0.01) but not short sleep (aOR 1.19, 95% CI 0.98 to 1.44, p=0.07) compared with non-users. Heavy users (≥20 of the past 30 days) were more likely to have both short sleep (aOR 1.64, 95% CI 1.28 to 2.09, p<0.001) and long sleep (aOR 1.76, 95% CI 1.19 to 2.60, p<0.01) compared with non-users (table 3). Compared with former cannabis users (no use in past 30 days), recent users were more likely to report both short sleep (aOR 1.34 95% CI 1.12 to 1.62, p<0.01) and long sleep (aOR 1.65, 95% CI 1.29 to 2.11, p<0.001).
Discussion
In this study, we demonstrate a significant association between recent cannabis use and the extremes of nightly sleep duration in a nationally representative sample of adults aged 20–59. There was also an exposure–response between cannabis use frequency and the prevalence of both short sleep (<6 hours per night) and long sleep (>9 hours per night). These results were not different between survey years.
The literature examining the effects of cannabis on sleep duration, architecture, or quality in people with no sleep disturbances at baseline demonstrate overall mixed results.5–8 Early polysomnographic studies suggest that even a single cannabis exposure can cause reduced sleep-onset latency, increased total sleep time, and less disruptions once asleep.21 Repeated use can quickly demonstrate habituation and likely opposite effects: increased sleep-onset latency, decreased total sleep time, and greater sleep disruption.22 While there is no clear temporal or frequency definition for acute versus chronic exposure, participants in our study who endorsed regular use were thought to represent chronic users. We determined there to be a possible exposure–response relationship between frequency of use and sleep duration; heavy users (cannabis use on 20 or more of the past 30 days) were at the greatest risk of both extremes of nightly sleep duration compared with non-users. This bimodal risk distribution has not previously been reported, particularly the association between cannabis use and long sleep. With our cross-sectional analyses, we can only speculate that these findings may be related to an unknown consequence of repeated cannabis exposure alone or may be a reflection of other underlying sociodemographic or health factors. While the doses used by NHANES participants were not reported, our results imply that frequency of use alone may impact sleep architecture. Further clinical study is needed to characterize the dose–response relationships between cannabis use and sleep outcomes.
There has been a surge of interest in cannabinoids such as cannabidiol (CBD) in recent years as potential sleep aids for primary sleep-wake disorders such as insomnia, restless leg syndrome, central sleep apnea, obstructive sleep apnea (OSA), as well as for sleep disturbances secondary to other conditions such as chronic pain or post-traumatic stress disorder (PTSD).7 8 In contrast to CBD, current evidence suggests that delta-9 tetrahydrocannabinol (THC), the other major cannabinoid present in most strains of cannabis, has stimulant and hallucinogenic properties contributing to sleep disruption.8 With an evolving market of new cannabis strains and cannabinoid formulations, each with varying proportions of CBD and THC, the generalizability of the current literature about sleep impact is limited. Despite insomnia being one of the most cited reasons for self-medication with cannabis or cannabinoids, the evidence base is overall inconsistent and of poor quality, as described in a recent systematic review.23 Currently, the only indications with some robust evidence for therapeutic cannabis use to improve sleep duration or quality are OSA, PTSD, and chronic pain syndromes.24–26
Increasing prevalence of both cannabis use and sleep deprivation in the population is a potential cause for concern. While our cross-sectional analysis did not demonstrate an association between recent cannabis use and daytime sleepiness, insufficient sleep in the modern world is a growing public health issue9 and sleep disturbances can be a major risk factor for initiating cannabis use.27 This can perpetuate cycles of increased cannabis use, progressive sleep disturbances, and acute cessation leading to withdrawal which may add further negative effects to sleep architecture and quality.28 The importance of adequate sleep is underscored by the known health consequences of chronic sleep deprivation and chronic oversleeping. Both states can predispose to traumatic injuries, cardiovascular comorbidities, depression, cognitive deficits, and pain.11–13 The extremes of sleep thus exhibit a ‘U-shaped’ relationship with morbidity and mortality at the individual and population levels.17 29 This is thought to be related to pathological states of generalized systemic inflammation with chronic short sleep, long sleep, or other disturbances.30 The role of the endocannabinoid system in exacerbating or alleviating these inflammatory pathways in the long term is not fully understood and an important area of ongoing study as cannabis use continues to grow in prevalence.
This study has several limitations inherent in its design that should be considered when interpreting the results. First, data collected from survey questionnaires are self-reported and therefore subject to selection bias; only persons willing and able to participate in the survey and examination process are included. In addition, the historical (and still present) stigma associated with cannabis may impact participant responses to questions about cannabis use. However, in our analyses, there was no significant interaction between survey year and cannabis use variables, suggesting that the effects of this response bias did not differ over the study period. Second, given the cross-sectional nature of our study there may be residual unmeasured confounding we are unable to account for, including confounding by indications for cannabis use. Furthermore, these cross-sectional data are unable to provide insight on causality (or reverse causality) between sleep outcomes and cannabis use. Third, granular data related to cannabis exposure (formulations, dosing, intended purpose of use) and sleep-related metrics (timing, quality, efficiency, alertness, or prior sleep disorders) were not available but would have allowed for further analysis. Fourth, the NHANES cannabis survey did not include the adolescent demographic, which represent the group at highest risk of both cannabis-related harms and sleep disturbances.31 Thus, the generalizability of our findings is limited.
Conclusions
Recent cannabis use was associated with the extremes of self-reported nightly sleep duration in this nationally representative sample of adults, with suggestions of a dose–response relationship. Despite the current literature demonstrating mixed effects of cannabis and various cannabinoid formulations on sleep architecture and quality, these agents are being increasingly used as both prescribed and unprescribed experimental therapies for sleep disturbances. Sleep-wake physiology and regulation is complex and research about related endocannabinoid pathways is in its early stages. Given the growing prevalence of cannabis use, future studies should continue exploring the relationship to human sleep using polysomnographic or actigraphic analysis. A better understanding of the endocannabinoid-mediated effects on sleep can inform development of clinical guidelines to target improved long term health outcomes at the patient and population levels, particularly for demographic groups that may be at increased risk of consequent sleep disturbances.
Data availability statement
Data are available in a public, open access repository. Data are available on reasonable request. All data for this study and tutorials to access the data are publicly available at https://wwwn.cdc.gov/nchs/nhanes/.
Ethics statements
Patient consent for publication
Ethics approval
The data collection protocols are approved by the NCHS Ethics Review Board and all survey participants provide informed consent prior to being interviewed and examined.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @calvdiep, @chenchentian, @kathak_v, @wonchristine, @drhaclarke, @mndpsingh7, @drladha
MS and KSL contributed equally.
Contributors CD, MS, and KSL conceived the study. CD conducted data retrieval, coding, and analysis. CD, CT, KV, CW, DNW, HC, MS, and KSL all contributed to drafting and preparation of the manuscript. KSL acts as a guarantor for all activities of this study.
Funding DNW, HC, MS, and KSL are supported in part by Merit Awards from the Department of Anesthesiology and Pain Medicine at the University of Toronto (Toronto, Canada). DNW is supported in part by a Endowed Chair in Translational Anesthesiology Research at St. Michael’s Hospital (Toronto, Canada) and the University of Toronto (Toronto, Canada). MS is supported by a Career Scientist Award from the Canadian Anesthesiologists’ Society (Toronto, Canada).
Competing interests HC and KSL are co-principal investigators for an observational study of medical cannabis use funded by Shoppers Drug Mart.
Provenance and peer review Not commissioned; externally peer reviewed.