Article Text
Abstract
Background There has been a surge in interest in radiofrequency ablation (RFA) of the genicular nerves over the past decade, with wide variability in selection, technique and outcomes. The aim of this study is to determine factors associated with treatment outcome.
Methods We retrospectively evaluated the effect of 23 demographic, clinical and technical variables on outcomes in 265 patients who underwent genicular nerve RFA for knee pain at 2 civilian and 1 military hospital. A primary outcome was designated as a > 30% decrease in average knee pain score lasting at least 3 months without cointerventions.
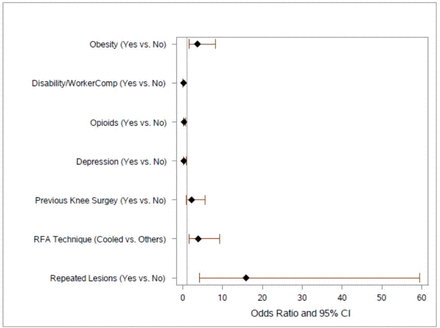
Results The overall rate of a positive response was 61.1% (95% CI 55.2% to 67.0%). In univariable analysis, larger electrode size (p=0.01), repeated lesions (p=0.02), having>80% pain relief during the prognostic block (p=0.02), not being on opioids (p=0.04), having no coexisting psychiatric condition (p=0.02), having a lower baseline pain score (p=0.01) and having >3 nerves targeted (p=0.02) were associated with a positive outcome. In multivariate logistic analysis, being obese (OR 3.68, 95% CI 1.66 to 8.19, p=0.001), not using opioids (OR 0.35, 95% CI 0.16 to 0.77, p=0.009), not being depressed (OR 0.29, 95% CI 0.10 to 0.82, p=0.02), use of cooled RFA (OR 3.88, 95% CI 1.63 to 9.23, p=0.002) and performing multiple lesions at each neural target (OR 15.88, 95% CI 4.24 to 59.50, p<0.001) were associated with positive outcome.
Conclusions We identified multiple clinical and technical factors associated with treatment outcome, which should be considered when selecting patients for RFA treatment and in the design of clinical trials.
- treatment outcome
- diagnostic techniques and procedures
- chronic pain
- pain management
- nerve block
Statistics from Altmetric.com
Introduction
Knee pain is a leading cause of disability worldwide,1 with a lifetime prevalence rate around 45%.2 Whereas the most common cause of knee pain is osteoarthritis, other etiologies include traumatic arthritis, inflammatory arthritis and persistent pain.3 4 The prevalence increases with age, obesity and a history of trauma,5–7 and is expected to rise in the coming decades.7
Conservative treatments, including joint injections, provide limited relief and may be associated with significant risks in an elderly population, including hyperglycemia and infection.5 8–10 Many of these patients are referred for surgery. In 2013, it was estimated that 4 million living Americans had received a total knee replacement, with over 600,000 performed annually.11 Joint replacements typically last between 10 and 20 years, with younger people increasingly offered the surgery. In those who undergo knee replacement, between 12% and 44% experience persistent pain, with 15% describing their pain as severe.4 12 13 These statistics indicate a strong need for non-surgical therapeutic alternatives.
In the past decade, there have been several randomized controlled trials that have evaluated radiofrequency ablation (RFA) for knee pain.14–20 These studies have generally enrolled small numbers of patients, typically targeted only three of more than 10 nerves that innervate the joint,21 often performed RFA without prognostic blocks,15 17 19 20 did not require radiographic imaging,18 included patients with prior surgery17 and used a wide variety of screening tests and RF techniques. Yet, most,14 18–20 but not all15 17 studies demonstrated benefit. The poor quality of studies has led to increased scrutiny from payers, and calls for higher quality studies based on refined selection criteria, more aggressive neural targeting, and evidence-based technical performance.5 22–26 The main objective of this study is to determine demographic, clinical and technical variables associated with RFA outcomes for chronic knee pain. We hypothesized that similar to RFA for other conditions, better selection would translate to superior outcomes.
Methods
Selection criteria
All procedures were performed between June 30 2013 and May 1 2019. Inclusion criteria were patients with a primary complaint of knee pain treated with a radiofrequency procedure(s). Excluded from consideration were individuals in whom adequate follow-up (>3 months) was not available; individuals without documentation of an objective means to assess outcome (eg, pain scores not recorded); and individuals who received a new concurrent treatment such as a regularly prescribed analgesic that could affect interpretation of treatment results.
Patients who met selection criteria were identified by searching electronic billing records or Relative Value Units (Walter Reed) databases for the current procedural terminology (CPT) code ‘64640’ (‘destruction by neurolytic agent, somatic nerve’) or 64 624 (‘destruction by neurolytic agent, genicular nerve branches’). Individuals identified by the procedure search were then cross-referenced using the International Classification of Diseases (ICD)-9 and ICD-10 codes for ‘pain in knee’ (M25 or 719.46), ‘osteoarthritis of knee’ (M17 or 715.16) and ‘osteoarthritis, unspecified site’ (M19.90 or 715.30/715.90).
Procedures
Prognostic blocks
Prognostic blocks were performed in the supine position, with the knee flexed at a 30°–60o angle under resting pillows. Light sedation with midazolam (≤2 mg), fentanyl (≤100 mcg) and/or ketamine (≤20 mg) was administered on an ‘as-needed’ basis to patients who could not tolerate the procedure with local anesthetic alone. All procedures were performed using fluoroscopic guidance in anterior and lateral views to ascertain needle placement. Unilateral procedures were performed in all patients, with only those obtaining significant relief from RF ablation receiving later treatment on their less painful knee, if indicated. Superficial anesthesia was administered via a 25- or 27- gauge needle using lidocaine 1%. In all patients who received prognostic blocks, 22- or 25- gauge spinal needles were inserted at the traditional locations for the superomedial, superolateral and inferomedial genicular nerves.14–17 19 20 Depending on the pain location, patient tolerability and physician preference, additional needles were sometimes placed at additional sites based on recent anatomical dissections.21 23–25 The locations for the targeted nerves were roughly as follows:
Superomedial genicular nerve: Electrode positioned superomedially at the femoral epicondyle, at about 60%–80% depth to the posterior border and 1–4 mm superficial to periosteum.
Nerve to vastus medialis: Same as superomedial genicular nerve, except electrode withdrawn to 33%–50% depth and positioned 0.5–1 cm off bone.
Superolateral genicular nerve: Electrode positioned superolaterally at the femoral epicondyle, at a similar depth and distance from bone as for the superomedial genicular nerve.
Inferomedial genicular nerve: Electrode positioned medially at the mid-border of the tibial condyle at approximately one half to two-thirds depth to the posterior border.
Inferolateral genicular nerve: Electrode positioned laterally at the lower border of the femoral epicondyle or upper border of tibial condyle at approximately one-half to 80% depth to the posterior border.
Recurrent fibular nerve: Electrode positioned along a longitudinal line caudad from Gerdy’s tubercle, 1 cm below tibial tuberosity at one-half to 80% depth.
Medial branches of nerve to vastus intermedius: Electrode placed 5 cm superior to the upper patella and 5 mm toward midline from the medial border of the femoral shaft, 1–4 mm superficial to the bone.
Nerve to vastus lateralis (NVL): Electrode placed 5 cm superior to upper patella and 5 mm toward midline from lateral border of femur, 0.5–1 cm superficial to the bone.
Lateral branches of nerve to vastus intermedius: Similar to landmarks for NVL, except electrode positioned deeper (1–4 mm superficial to bone).
Infrapatellar branch of the saphenous nerve: Electrode placed along a longitudinal line 4 cm medial to apex of patella and tibial tubercle. For those with inferomedial quadrant the lesion(s) was performed in the inferior segment, while those with diffuse or superomedial pain had a lesion done in the superior segment, just below and above the joint line, respectively.
After negative aspiration at each site, a 0.5–2 mL solution of bupivacaine 0.5% was then injected. In the recovery area, patients were given a 6-hour pain diary to return the following day, and instructed to discount procedure-related pain and engage in their typical activities. A positive block was defined as >50% pain relief that lasted for at least 3 hours.
Radiofrequency treatment
RF ablation was performed in the supine position, with the targeted knee positioned similarly to the prognostic blocks. The same nerves targeted for the screening blocks were treated in the RF procedure. Superficial anesthesia and sedation as needed were administered in a similar fashion to that used for the prognostic blocks. The electrodes were inserted in coaxial views (eg, directed slightly posteromedially for the superomedial and superolateral genicular nerves) until periosteum was contacted at the targeted locations described under ‘prognostic blocks’, then withdrawn slightly so that they were approximately 1–10 mm off bone, depending on the nerve being targeted (eg, more proximal for the nerves to the vastus medialis and lateralis), stimulation results, RF technique (ie, cooled vs conventional RFA, active tip size, angle inserted), body habitus and provider preference, see review.26 At each neural target, electrodes were adjusted to optimize sensory stimulation at 50 Hz, with the goal being concordant sensation at <0.7 V, which is consistent with the National Institutes of Health-sponsored Sequenced Strategy for Improving Outcomes in People with Knee Osteoarthritis Pain (SKOAP) trial for knee osteoarthritis.27 Once position was optimized, motor stimulation was used to verify the absence of distal muscle contractions. After ideal electrode placement was ascertained, 1 mL of 2% lidocaine was injected to reduce procedure-related pain and enhance lesion size.28 The three types of RF performed were conventional ablation, ‘cooled’ ablation using internally cooled electrodes and pulsed (non-ablative) RF treatment. Conventional and pulsed RF were performed with straight 18–20- gauge 100–145 mm radiofrequency needles with 10 mm active tips, while cooled RF was accomplished with 17-gauge, 75 mm cooled electrodes with 4 mm active tips (Coolief, Avanos Medical, Alpharetta, Georgia, USA). For conventional RF, the temperature was set at either 80o or 90°C for 75–150 s, whereas the default generator settings for cooled RF were a temperature of 60°C with a 150 s lesion time, which creates tissue temperatures greater than 80℃. When pulsed RF was performed, the parameters for treatment were: voltage output 40–60 V; 2 Hz frequency; 20 ms pulses in a 1 s cycle, 120 s duration per cycle with one or two cycles per target site; target impedance range between 150 and 400 Ohms; and 42°C plateau temperature.29 Following ablative treatment, 2–10 mg of depomethylprednisolone was injected per lesioning site to minimize the risk of neuritis.28 In the recovery area, immediate complications were surveilled, and patients on analgesic medications were instructed on how to taper these medications.
Outcome measures
The covariates included a range of demographic, clinical and technical factors that were selected due to their demonstrated effect on outcomes for the interventional treatment of knee osteoarthritis and for radiofrequency procedures involving other joints.28 Baseline data collected included age, sex, duration of pain, etiology and diagnosis, analgesic medications, prior surgery (defined as any surgery intended to repair structural damage (including ligament and meniscal surgery) and excluding diagnostic arthroscopy, skin lesion removal or joint aspiration), medical and active (eg, receiving medications, counseling or involved in a 12-step program) psychiatric comorbidities (eg, affective disorders, anxiety, substance misuse disorder, post-traumatic stress disorder), degree of joint degeneration, social variables (disability status, smoking, work injury), location of pain, technical factors such as the results of prognostic blocks, type of radiofrequency, temperature, lesioning time, number of nerves targeted and whether multiple lesions were performed at different locations for a single target site. Variables including psychiatric comorbidities, radiolographic degeneration, coexisting pain conditions, presence and type of surgery, opioid use and social variables were obtained from manual review of electronic health records and imaging studies. In all individuals, the three nerves targeted in the randomized trials by Choi et al and others were treated.14–17 19 20 30 Subsequent nerve targets as described above were tabulated numerically (ie, five nerves equated to two additional target sites).
The primary outcome measure was procedural success, with a positive categorical outcome designated as >30% pain relief lasting at least 3 months, without intervening interventions.31 All patients in the study were followed up 4–6 weeks after the procedure, with most at or after 3 months. A positive outcome was predefined as a reduction in Numerical Rating Scale (NRS) pain score>2/10 without concomitant interventions at the first follow-up visit at or after 3 months. If the patient had a positive outcome at their 4–6 weeks visit and was not seen again before 6 months but returned for a repeat procedure reporting prolonged relief lasting greater than 3 months (n=7), this was also designated to be a positive outcome. The percent pain reduction was calculated based on the difference between the 3 months (or first follow-up visit after) and the baseline average knee pain score on a 0–10 NRS. In individuals with bilateral pain who underwent a second procedure on a subsequent visit, only the results of the first (most painful) procedure were tabulated. Patients who had a positive outcome at 4–6 weeks but were subsequently lost to follow-up were excluded from analysis (figure 1).
Flow chart showing study subject disposition. CPT, current procedural terminology; RFA, radiofrequency ablation.
Statistical analysis
We constructed descriptive statistics in the form of medians and quartiles for ordinal and continuous variables, and in the form of frequency tables for categorical variables. We applied univariable logistic regression analyzes with each baseline variable as a regressor in predicting the outcome of procedure success. We then applied a multivariable logistic regression analysis via a stepwise selection process with a significance level of entering the model equal to 0.15 and a significance level of staying in the model equal to 0.15. The initial stage of the stepwise selection process included those regressors with a p<0.15 that had less than 33% missingness. Fourteen variables were entered into the model (age, sex, baseline pain score, obesity, disability or worker’s compensation, opioid use, depression, previous knee surgery, Kellegren-Lawrence grade >2, number of nerves targeted, prognostic block volume>0.5 mL, RFA techniques, needle size <20-gauge, repeated lesions), with seven being selected as most explanatory (table 1 and figure 2). We also constructed a receiver operating characteristic curve based on the final multivariable model. We invoked a significance level of 0.05 for the univariable logistic regression analyzes and did not impose any multiplicity adjustments. We used SAS V.9.4 for all statistical analyses.
Summary of stepwise selection of regressors
Forest plot: factors associated with successful outcome (multivariable analysis). RFA, radiofrequency ablation.
Results
Baseline demographic and clinical information
Eight hundred and thirty-one patients were identified as having undergone procedures using the CPT and ICD search codes. From this cohort, 297 individuals underwent RFA of the genicular nerves and 265 patients had outcomes available for analysis, of which 61.1% experienced a positive outcome (figure 1). There were 96 males and 169 females in the final data set. The mean age of subjects was 64.3 years (SD 15.2), and the average duration of pain was 7.1 years (SD 6.8). These individuals had a mean baseline NRS pain score of 6.9 (SD 2.0), 39.1% had prior knee surgery (52.9% of whom underwent knee arthroplasty), 34.2% were on opioids, 33.3% had a concomitant psychiatric illness and 51.4% had concomitant pain conditions.
Demographic and clinical factors associated with outcome
Demographic and clinical factors associated with outcome are reported in table 2. The mean baseline pain score among patients with positive outcomes was lower than those with negative outcomes (6.6±2.0 vs 7.3±SD 1.9, p=0.014). Patients on opioids were less likely to experience meaningful relief from their procedure than opioid-naïve patients (52.4 vs 66.7% success rate, p=0.040). Patients with depression were less likely to experience a positive RFA outcome than those with no active psychiatric illness (38.3% vs 64.3%, p=0.003), though other psychiatric comorbidities were not associated with outcome. There were no significant differences in the outcome with respect to age, sex, smoking status, obesity, prior surgery (no=56.6%, yes=68.6%, p=0.053), prior total knee arthroplasty (non-TKA=79.1%, TKA=63.0%, p=0.09) or disability status table 2.
Demographic and clinical characteristics by outcome
Technical factors associated with outcomes
Technical factors associated with outcome in univariable analysis are shown in table 3. Individuals who had >3 nerves targeted fared better than those who underwent RFA at only the superomedial, superolateral and inferomedial genicular nerve targets (80.0% vs 57.5%, p=0.02). Creating more than one lesion per nerve resulted in a higher proportion of successful procedures than limiting RFA to a single cycle (78.9% vs 58.7%, p=0.02). The utilization of cooled RFA was associated with a higher success rate than using pulsed or conventional RFA (67.5% vs 54.5%, p=0.049 compared with conventional), and using 18-gauge or larger electrodes resulted in a higher success rate than using smaller electrodes (66.1% vs 50.0%, p=0.01). When techniques associated with set protocols (eg, cooled RF with 17-gauge electrodes, pulsed RF with 20-gauge electrodes) were excluded from analysis, neither electrode size (53.6% success rate for electrodes larger than 20-gauge vs 54.1% for smaller, p=0.97) nor lesion duration (60.0% success rate for lesion duration<120 s vs 52.1% for>120 s, p=0.48) was associated with RFA outcome. Compared with the 11 people who had less than 50% pain relief with prognostic blocks (9.1% success rate), those who obtained 50%–79% relief (48.6% success rate, p=0.042), and who reported at least 80% relief (63.5% success rate, p=0.008) experienced better outcomes. The use of prognostic block volumes <0.5 mL fell shy of statistical significance compared with volumes >0.5 mL (p=0.053).
Treatment characteristics by outcome
Multivariable analysis
The results of multivariable analysis are shown in figure 2. In multivariate logistic analysis, being obese (OR 3.68, 95% CI 1.66 to 8.19, p=0.001), not using opioids (OR 0.35, 95% CI 0.16 to 0.77, p=0.009), not being depressed (OR 0.29, 95% CI 0.10 to 0.82, p=0.02), use of cooled RF (OR 3.88, 95% CI 1.63 to 9.23, p=0.002), and performing multiple lesions at each neural target (OR 15.88, 95% CI 4.24 to 59.50, p<0.001) were associated with positive outcome. Having prior knee surgery (OR 2.21, 95% CI 0.88 to 5.55, p=0.09) and not being on disability (OR 0.20, 95% CI 0.04 to 1.04, p=0.056) were not statistically significant due to collinearity with other variables.
Discussion
Key findings
The key significant findings in this multicenter study are somewhat intuitive based on previous research on chronic pain treatment: that not having a coexisting psychiatric condition, not being on opioids, having less baseline disease burden (ie, lower pain score), targeting more nerves, using strategies to ablate greater surface areas (eg, using larger electrodes and creating multiple lesions), and having greater pain relief on the prognostic block, were positively associated with treatment outcome. Although we found some unusual trends (eg, obese patients and those with prior non-arthroplasty surgery were more likely to experience a positive outcome), these fell shy of statistical significance. Whereas individuals who have undergone arthroplasty should theoretically not experience pain from joint degeneration, they may still perceive soft-tissue pain transmitted through small branches targeted by a more aggressive lesioning strategy (eg, nerves to the vastus lateralis and medialis, infrapatellar branch of the saphenous nerve).
Flaws with previous studies
Over the past decade, the use of genicular nerve RFA for knee pain has soared, culminating in numerous clinical studies, a conditional recommendation by the American College of Rheumatology in 2019, and new billing codes from the Centers for Medicare and Medicaid Services.32 Although early randomized trials demonstrated benefit from genicular nerve RFA, these studies were small and methodologically flawed. These flaws are perhaps best illustrated by the first and most cited publication by Choi et al,14 who used excessive block volumes, created small lesions, likely selected placebo responders by requiring>24 hours of pain relief with prognostic blocks using lidocaine, and targeted only three of more than a dozen nerves providing nociceptive input from the knee joint. Recently, numerous cadaveric studies have called into question the effectiveness of targeting only three nerves, and the validity of the previous anatomical targets.21 23–25
Comparison with other studies: expected findings
Previous studies have examined factors associated with outcome for RFA procedures performed at other locations, and for non-RFA interventions. Similar to our study, they have generally found that greater disease burden (eg, higher pain scores) and concomitant psychiatric conditions are associated with poorer outcomes, for procedures and non-interventional treatments.28 33–35 For psychiatric conditions, depression, but not other psychological illnesses, portended a negative outcome. We found that opioids were associated with treatment failure in multivariable analysis, which is consistent with multiple other studies evaluating surgical and non-surgical procedures, including RFA.36–38 Reasons why opioid use may predispose to treatment failure include lower pain thresholds and poorer tolerance, unrealistic expectations, hyperalgesia and secondary gain. Perhaps the least surprizing finding was that treating more nerves was associated with superior outcomes, which prima fascie underscores the basis for RFA (ie, interrupting nociceptive input will reduce pain).
Larger electrodes, cooled RF and creating multiple lesions were all associated with positive RFA outcome. What these strategies have in common is that all create larger lesions, thereby reducing the likelihood of ‘missing nerves’, a common cause of treatment failure.39 Consequently, guidelines on the use of RFA to treat facet arthropathy recommend the use of large electrodes and consideration of multiple lesions when sensory stimulation is not used or insufficient.28
Although there was a high percentage of missing data, greater pain relief during prognostic blocks was associated with better treatment outcomes. Whereas the facet guidelines recommend using a 50% cut-off for selecting patients for RFA due to concerns about an increased false-negative rate with higher thresholds, they acknowledge that greater success rates are likely to be achieved with higher cut-off values.28 However, unlike lumbar facet joint pain whereby the blocks confer diagnostic value, the benefit of prognostic blocks is unclear.28andomized studies evaluating genicular nerve RFA have generally reported a greater than 80% positive rate for local anesthetic blocks, and a controlled study found no significant difference in success rates when patients underwent RFA without a block (64%) vs only after a positive block (59%).5 30 Therefore, based solely on these results, we cannot advocate a higher prognostic block cut-off rate.
Limitations
There are several limitations to our study which warrant consideration. First, this study is subject to the same methodological drawbacks as other retrospective studies, which include missing data, non-standardized reporting and variations in procedural performance. Second, the non-blinded nature of the study likely overestimated effectiveness, particularly for parameters that might have affected expectations such as the use of stimulation and targeting more nerves. Last, because secondary outcome measures such as validated instruments measuring quality of life were not routinely used, our definition of success was limited to subjective pain reduction, which is only one of several core outcome domains.31
Conclusions
In this study, we identified several demographic, clinical and technical factors that may affect treatment outcomes for knee RFA. Some factors typically associated with poor outcomes such as disability were not associated with outcome in multivariable analysis, while others such as prior knee surgery and obesity were actually associated with treatment success when controlling for confounding variables. Similar to spinal RFA procedures, techniques that enhance lesion size and reduce the chance of missing target nerves may increase the likelihood of treatment success.
References
Footnotes
Contributors All of the authors have contributed to the planning, conducting and reporting of the work described in the article.
Funding Funded in part by a grant from MIRROR, Uniformed Services University of the Health Sciences, US Dept. of Defense, and grant # HU00011920011.
Competing interests SPC has served as a consultant for Avanos (Alpharetta, GA) in the past 2 years.
Patient consent for publication Not required.
Ethics approval Permission to conduct this study was granted by the Institutional Review Boards of Johns Hopkins and Penn State-Hershey Medical Center, the Board of Directors at the Pain Management Institute, Bethesda, MD and Washington, DC, and the Walter Reed Dept. of Investigation.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available on reasonable request. Deidentified participant data.