Article Text
Abstract
Background and objectives Obtaining consistent efficacy beyond 12–24 hours with local anesthetics, including extended-release formulations, has been a challenging goal. Inflammation resulting from surgery lowers the pH of affected tissues, reducing neuronal penetration of local anesthetics. HTX-011, an investigational, nonopioid, extended-release dual-acting local anesthetic combining bupivacaine and low-dose meloxicam, was developed to reduce postsurgical pain through 72 hours using novel extended-release polymer technology. Preclinical studies and a phase II clinical trial were conducted to confirm the mechanism of action of HTX-011.
Methods In a validated postoperative pain pig model and a phase II bunionectomy trial, the analgesic effects of HTX-011, oral meloxicam (preclinical only), liposomal bupivacaine (preclinical only) and saline placebo were evaluated. The optimal meloxicam:bupivacaine ratio for HTX-011 and the effect of HTX-011 on incisional tissue pH were also evaluated preclinically.
Results Preclinical data demonstrate the ability of HTX-011 to address local tissue inflammation as demonstrated by a less acidic tissue pH, which was associated with potentiated and prolonged analgesic activity. In the phase II bunionectomy study, HTX-011 achieved superior and sustained pain relief through 72 hours after surgery compared with each component in the polymer.
Conclusions Preclinical animal and clinical results confirm that the low-dose meloxicam in HTX-011 normalizes the local pH in the incision, resulting in superior and synergistic analgesic activity compared with extended-release bupivacaine. HTX-011 represents an extended-release local anesthetic with a dual-acting mechanism of action that may provide an important advancement in the treatment of postoperative pain.
Trial registration number NCT02762929.
- postoperative pain
- pharmacology: local anesthetics
- pain medicine
- animal studies
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, an indication of whether changes were made, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
The management of acute postoperative pain continues to pose a significant challenge to clinicians across all surgical specialties, with the most severe pain after surgery occurring in the first 72 hours.1 Despite the administration of opioid and nonopioid analgesics during this timeframe, most patients still report that their pain is poorly managed.2 The use of opioids is associated with an increased risk of opioid-related adverse events such as nausea, vomiting, respiratory depression and sedation. Along with uncontrolled pain, these adverse events have been shown to contribute directly to increased length of hospital stay and medical costs.3 More effective nonopioid analgesics are needed to reduce overreliance on opioids when managing postoperative pain and limit risks of abuse, addiction and diversion.
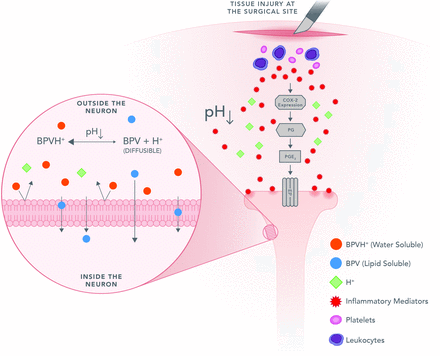
Local anesthetics represent an important alternative to the use of systemic opioids, with bupivacaine hydrochloride (HCl), an immediate-release amide-type anesthetic, as the most widely used for addressing postoperative pain.4 However, bupivacaine HCl has a limited duration of action (≤12 hours). Recent attempts to extend the delivery of bupivacaine HCl by using extended-release (ER) technology have failed to demonstrate consistent clinical activity beyond 24 hours despite evidence of extended drug release by prolonged plasma concentrations.5 The decreased activity of local anesthetics in the presence of inflammation is a well-described phenomenon.6 Increased tissue acidity caused by the normal inflammatory process from injury, infection or surgical intervention reduces the penetration of local anesthetics (both amide and ester classes) such as bupivacaine into nerve cells in the affected area,6 leading to diminishing analgesia beginning in the first few hours and lasting for several days (figure 1). In addition, inflammatory mediators can also directly act on nociceptors, leading to peripheral sensitization that can further contribute to reduced local anesthetic activity.7
Mechanism of bupivacaine penetration into the nerve. BPV, bupivacaine.
HTX-011 is an investigational, ER, fixed-dose, dual-acting local anesthetic that contains two active ingredients, bupivacaine and low-dose meloxicam. Meloxicam was included to reduce the local inflammatory response to tissue injury from surgery in order to maintain physiologic pH in the microenvironment at the surgical site and reduce cytokine-induced peripheral sensitization. To achieve an ER profile for both bupivacaine and meloxicam, HTX-011 uses a novel polymer technology,8 which provides simultaneous diffusion of the active ingredients in a controlled manner over 72 hours. Since the polymer formulation is somewhat viscous and hydrophilic, HTX-011 can be applied to the affected tissue in the surgical incision without a needle and remains where it is placed, releasing both drugs simultaneously through 72 hours.
Here, we present results from a preclinical postoperative pain model in pigs demonstrating that the incorporation of meloxicam in combination with bupivacaine in an ER formulation synergistically potentiates the activity of bupivacaine for 72 hours. These findings were evaluated in a phase II clinical study in bunionectomy that confirmed the synergistic mechanism of action of HTX-011.
Methods
Extended-release polymer formulations
Initial preclinical studies were conducted using polymer formulations with a 144-hour (6-day) drug release profile (table 1). Liposomal bupivacaine (EXPAREL, Pacira Bioscience, Parsippany, New Jersey, USA) and saline placebo were used as positive and negative controls. Once the impact of local inflammation on the analgesic activity of ER bupivacaine formulations and the mitigating effect of meloxicam on the activity of bupivacaine was recognized, formulations were optimized to provide a 72-hour (3‑day) release profile to address the period after surgery known to be most painful.1 For the purposes of comparing the combination to individual active ingredients, comparable ER formulations containing either bupivacaine alone or meloxicam alone were also prepared. Finally, multiple formulations of bupivacaine plus meloxicam with varying ratios were prepared and evaluated, resulting in the identification of the final composition of HTX-011. In the clinical studies, polymer formulations of bupivacaine alone (HTX-002) and meloxicam alone (HTX-009) were compared with HTX-011.
Preclinical treatments
Preclinical
This study was performed following approval of an application form submitted to the Committee for Ethical Conduct in the Care and Use of Laboratory Animals.
Postoperative pain model
An established animal model of postoperative pain in pigs was used to evaluate the ability of meloxicam to suppress surgery-induced local inflammation and normalize tissue pH, in order to potentiate the analgesic activity of bupivacaine.9 Pigs are used in the model because of their similarities to humans—they have similar innervation patterns of their skin and their pain response is predictive of pain measures observed in postoperative patients. The study drug (1.8–3.4 mL depending on the formulated concentrations of active ingredients) was administered in each treatment group (n=4) subcutaneously into the margins of a 3-cm incision made into the pig flank to produce incisional pain similar to that of a surgical procedure (online supplementary figure 1). Evaluation of pain was conducted as described in Castel.9 Briefly, the intact side (contralateral to the incised side) was tested first as a control measure. Von Frey filaments (1–60 g) were applied approximately 0.5 cm proximal to the skin incision three times with intervals of 5–10 s. If withdrawal by the animal from the stimulus did not occur, a thicker filament was applied, but if withdrawal occurred, a thinner filament was applied. Greater force to elicit a withdrawal response implies less nociceptive sensitivity, which was considered to be due to greater analgesia from the test formulation. Postoperative pain, as assessed by von Frey filament testing, was reported as the force required to elicit a withdrawal response. Von Frey filament testing was administered at baseline and at 1, 3, 5, 24, 48, and 72 hours postdose (assessments at 96, 120, and 144 hours postdose were included for the evaluations of the early 6-day release formulations).
Supplemental material
Investigation of meloxicam/bupivacaine ratios
Polymer fixed-combination formulations with ratios of meloxicam to bupivacaine ranging from 0 to 0.06 were prepared and evaluated in pigs to identify the minimum ratio for clinical use. The log of the average von Frey withdrawal force over 24–72 hours values was plotted against the meloxicam/bupivacaine ratio and fit to a sigmoidal Emax model. The final, optimized formulation of the combination was designated HTX-011.
Evaluation of meloxicam site of action
To investigate if the effect of meloxicam was locally mediated or systemically mediated, an additional group of pigs (n=4) was administered a 3-day release ER bupivacaine formulation (ER-B-3D; table 1) plus a supratherapeutic dose (twice the maximum approved human dose) of oral meloxicam (Loxicom Oral Suspension, Norbrook, Overland Park, Kansas, USA) 0.5 mg/kg once daily on days 1 through 6.
Effect of HTX-011 on local pH
The effect of HTX-011 on the pH of the tissues at the surgical incision was also evaluated in the same pig postoperative pain model to confirm the underlying mechanism of action. Incisions were made on the left and right flank of each of 4 pigs. The left flank was treated with HTX-011 and the right flank was left untreated (sham control). Tissue pH at the incision was measured using needle pH electrodes (Microelectrodes, Bedford, New Hampshire, USA) embedded into the subcutaneous tissues for 48 hours.
Clinical
The safety and efficacy of HTX-011, matching polymer formulations containing only bupivacaine (HTX-002) or only meloxicam (HTX-009), and saline placebo (negative control) were evaluated in a phase II bunionectomy study (ClinicalTrials.gov: NCT02762929, registered May 2016). The study protocol and all amendments were approved by a centralized institutional review board (Western Institutional Review Board, Puyallup, Washington, USA) and the initial protocol was also approved by a local institutional review board (CHRISTUS Health IRB, Irving, Texas, USA). All subjects provided written informed consent prior to participation in any study-specific procedures. Inclusion and exclusion criteria are included in online supplementary table 1. Eligible subjects underwent a unilateral simple Austin-type bunionectomy under regional anesthesia with no more than 20 mL of 1% lidocaine without epinephrine administered as a Mayo block. For the purposes of comparing HTX-011 to its individual components and the controls, subjects were randomly assigned to receive a single dose by instillation into the wound of one of the following: HTX-011 (120 mg bupivacaine/3.6 mg meloxicam), HTX-002 (120 mg bupivacaine), HTX-009 (3.6 mg meloxicam), or saline placebo.
All subjects were required to remain in the clinic for assessments for 72 hours postdose and received opioids as rescue medication for pain control as needed. Pain intensity was assessed in each subject using an 11-point Numeric Rating Scale (NRS), where 0 equals no pain and 10 equals worst pain imaginable, and analyzed using the area under the curve (AUC) of pain intensity scores for each interval. The AUC was analyzed using analysis of variance. Endpoints for the study included AUC for pain intensity scores through the first 24, 48, and 72 hours postsurgery. X-rays to evaluate bone healing were performed.
Results
Preclinical studies
A 6-day ER bupivacaine formulation, ER-B-6D, was first compared with a positive control, liposomal bupivacaine, and a negative control, saline placebo. Liposomal bupivacaine demonstrated analgesic activity on day 1, but von Frey scores were similar to those of saline by 48 hours (figure 2), consistent with published clinical results.5 Administration of ER-B-6D resulted in greater analgesia, with a similar pattern of diminishing analgesia after 24 hours, reaching its nadir at 96 hours. There was a return of analgesic activity by 120 hours that further increased at 144 hours. As the period of diminished activity of ER-B-6D coincided with the expected time course of inflammation associated with tissue injury, combinations of bupivacaine and a nonsteroidal anti-inflammatory drug (NSAID) were evaluated with the goal of reducing local inflammation at the surgical site in order to maintain analgesic activity from 12 to 120 hours. Testing of a 6-day ER combination formulation of bupivacaine and meloxicam, ER-B/M-6D, resulted in a sustained analgesic effect that lasted from the time of administration through 144 hours (figure 2).
Analgesic effect of an ER bupivacaine/meloxicam combination formulation (ER-B/M-6D) compared with an ER bupivacaine formulation (ER-B-6D), liposomal bupivacaine, and saline placebo in a pig postoperative pain model. ER, extended release.
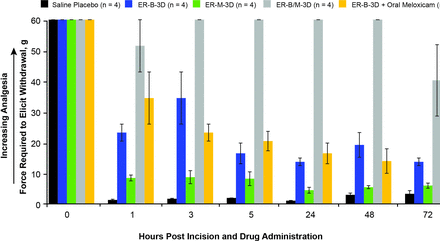
To assess the relative contributions of its components, the analgesic efficacy of a 3-day release formulation of bupivacaine and meloxicam, ER-B/M-3D, was compared with identical formulations of the individual active ingredients, ER-B-3D and ER-M-3D. ER-B/M-3D produced a substantially greater analgesic effect and maintained it over a longer period than either ER-B-3D or ER-M-3D (figure 3). ER-B-3D demonstrated moderate analgesia during the first few hours after surgery but this decreased and remained low from hour 5 through 72 as evidenced by lower values in tolerated maximal force in von Frey fiber testing. By contrast, from 24 hours and through 72 hours postdose, pigs that received the ER-B/M-3D tolerated a greater maximal force (40.3–60.0 g) compared with those that received ER-B-3D (13.8–19.3 g) or saline placebo (1.1–3.4 g). ER-M-3D provided almost no analgesia throughout the entire study period in these animals.
Analgesia from pig model after surgical incision through 72 hours following administration of ER bupivacaine plus meloxicam, ER meloxicam, ER bupivacaine with and without oral meloxicam, or saline placebo. ER, extended release.
The results observed with the ER-B/M-3D formulation led to a follow-up study to determine whether the effect of meloxicam on the activity of bupivacaine required local delivery or could be achieved with systemic administration. In a group of pigs receiving ER-B-3D and supratherapeutic daily doses of oral meloxicam (n=4), von Frey fiber results were similar to those for ER-B-3D alone, demonstrating that meloxicam must be administered locally in combination with bupivacaine to produce the optimal effect (figure 3).
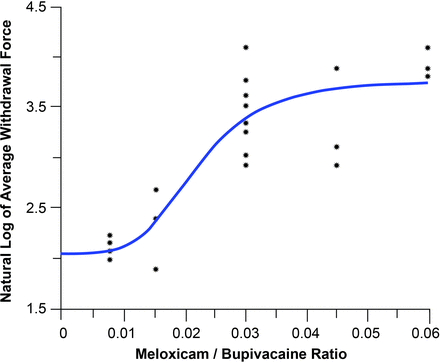
These results led to a full assessment to select the optimal ratio of bupivacaine and meloxicam. The relationship between the average von Frey withdrawal force and the meloxicam/bupivacaine ratio is presented in figure 4. The half-maximal effect was at a meloxicam/bupivacaine ratio of 0.022. Administration of formulations with a meloxicam/bupivacaine ratio of <0.030 generally resulted in a lower analgesic effect compared with formulations with ratios of ≥0.030. Thus, the lowest effective meloxicam/bupivacaine ratio was determined to be 0.030, and concentrations of 29 and 0.9 mg/mL of bupivacaine and meloxicam, respectively, were selected for further development in a formulation designated HTX-011.
Relationship between the average von Frey withdrawal force and meloxicam:bupivacaine ratio.
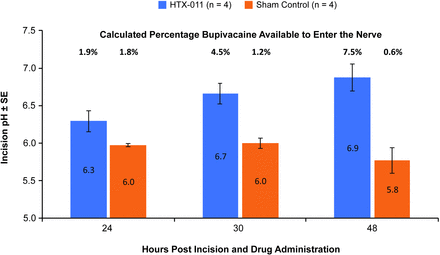
To confirm the proposed mechanism of action, tissue pH at the surgical incision was measured after administering HTX-011 into the margins of a surgical incision in pigs. In the pig model, tissue pH at the site of incision with HTX-011 treatment was consistently higher from 24 hours through 48 hours post-incision compared with control (figure 5). The approximately 1 pH unit difference observed at 48 hours relates to substantially more unionized bupivacaine available to enter the nerve with HTX-011 treatment compared with control (7.5% unionized vs 0.6% unionized, respectively).
pH of untreated or HTX-011-treated incisional tissue in pig postoperative pain model. SE, standard error.
Clinical evaluation of HTX-011 and its components
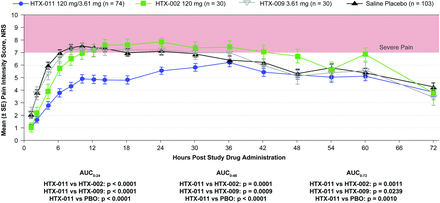
In the phase II clinical study, 237 subjects undergoing bunionectomy were included in the assessment of the individual components; 74 received HTX-011, 30 received HTX-002, 30 received HTX-009, and 103 received saline placebo. Subject characteristics were similar across the treatment groups; subjects were predominately white women with a mean age of 50 years (online supplementary table 2).
As shown in figure 6, subjects who received HTX-011 after undergoing bunionectomy exhibited significantly lower pain scores over the first 24, 48, and 72 hours (AUC0-24, AUC0-48, and AUC0-72) than those who received ER formulations of bupivacaine alone (HTX-002), meloxicam alone (HTX-009), or saline placebo. Pain curves for HTX-011 and HTX-002 separated within the first 5 hours. Similar to the preclinical model, the initial analgesic activity following administration of HTX-002 rapidly diminished and was comparable to placebo by 12 hours. Clinical results with HTX-009 were also similar to the preclinical model and indistinguishable from saline placebo.
Mean pain intensity scores through 72 hours following administration of HTX-011, HTX-002, HTX-009, or saline placebo in phase II bunionectomy study. AUC0-XX, area under the curve from 0 to XX number of hours. NRS, numeric rating scale; PBO, placebo; SE, standard error.
Although the study was not powered to detect a reduction in opioid use between groups, HTX-011 significantly increased the time to first opioid rescue and decreased opioid consumption compared with the HTX-009, HTX-002, and saline placebo groups (table 2). The overall incidence of any adverse event and the types of adverse events with HTX-011 were generally comparable to or lower than those for HTX-002 and HTX-009. Based on the X-ray results, there was no evidence of delays in bone healing in any treatment group.
Opioid consumption by treatment group
Discussion
Local anesthetics are used in most surgical procedures in the USA for postoperative pain management; however, consistent activity through the critical first 72 hours after surgery has been challenging. One barrier to developing a long-acting local anesthetic for treating postoperative pain is the ability of the local anesthetic to remain active in the local inflammatory, acidified environment that occurs as a result of surgery.10 The hypothesis that diminished local anesthetic response can be prevented by local delivery of meloxicam to control inflammation is supported by the results presented here. HTX-011 normalized tissue pH and significantly improved analgesic activity and duration of analgesia compared with its individual components.
Causes for diminished local anesthetic activity
The inflammatory process following surgery-associated tissue injury initiates several changes in the local tissue environment. The first action taken by the body immediately after tissue injury is to control bleeding.11 Platelets activate as part of the coagulation cascade and as the clot forms, they release cytokines that initiate the inflammatory response.12 Shortly after tissue injury, inducible cyclo-oxygenase (COX)-2 is upregulated13 and initiates a cascade of events that results in synthesis of proinflammatory prostaglandins such as prostaglandin E2 (PGE2). Serotonin released by platelets as well as the increasing concentrations of PGE2 in the presence of bradykinin results in an increase in local vascular permeability.14 This, coupled with the concentration gradient of chemotactic agents such as complement factors, interleukin-1, tumor necrosis factor-α, transforming growth factor p, and platelet factor 4, stimulates neutrophils to migrate into the wound.15 In addition, inflammation leads initially to a drop in pH due to local vasoconstriction and resulting ischemic conditions; later as a result of infiltration and activation of neutrophils in the tissue,11 there is increased energy and oxygen demand and accelerated glucose consumption via glycolysis. These conditions result in increased lactic acid secretion,16 17 causing acidification.18 Therefore, the low tissue pH observed is likely due to a combination of ischemia and activated leucocytes consuming oxygen and releasing acid.
Local anesthetics bind to the sodium voltage-gated ion channels from the interior of the neuron via the ionized (protonated) form. The pKa (pH where 50% of the molecules are unionized and 50% are ionized) of all local anesthetics is above the physiologic pH of 7.4. Therefore, when infiltrated into normal tissue, the majority of local anesthetic molecules are in the ionized state. However, in order to pass through the neuronal membrane, local anesthetics must be unionized19; thus, only a fraction of the local anesthetic molecules are able to pass through the neuronal membrane (figure 1). Once the unionized form enters the nerve, the equilibrium between unionized and ionized is reestablished and ionized molecules are available to bind to the voltage-gated ion channels.
Inflammation resulting from injury produces an acidic tissue environment outside the nerve favoring the ionized form, reducing the concentration of molecules available to penetrate into the nerve. This effect may explain why even as recent attempts to extend the delivery of local anesthetics have resulted in prolonging exposure, they have not demonstrated consistent pharmacodynamic activity beyond 24 hours.
An emerging line of evidence suggests that peripheral sensitization may also play a role in reducing the activity of local anesthetics in the presence of inflammation. PGE2 is known to act directly on C-fibers, resulting in a reduced action potential threshold leading to peripheral sensitization.20 One pathway by which this occurs is PGE2 binding to the EP class of receptors on the surface of the nerve, ultimately resulting in upregulation and increased excitability of tetrodotoxin-resistant ion channels, some of which appear to have low sensitivity to inhibition by local anesthetics.21 22
Development of HTX-011
HTX-011 utilizes a novel fourth-generation tri(ethylene glycol) poly(orthoester) (TEG-POE)-based formulation that is designed for parenteral, sustained-release drug delivery applications.8
TEG-POE polymer formulations are semiviscous liquids that control drug release via diffusion. The drug release rate from these formulations can be adjusted to provide delivery over a period of days to weeks by controlling the composition and molecular weight of the TEG-POE as well as the selection of biocompatible solvent excipients, such as the dimethyl sulfoxide and glycerol triacetate used in HTX-011.23 Because drug release is controlled by diffusion as opposed to polymer erosion used in other ER systems, there is no delayed early phase release, preventing the initial delivery of the active ingredient.23 Additionally, TEG-POE hydrolysis is slow during the delivery phase, ensuring that the active ingredients diffuse at a consistent rate. TEG-POE polymer is composed of repeating units containing a diol connected to an orthoester. Once the active components have been released from the formulation, the rate of polymer hydrolysis rapidly increases via the cleavage of the orthoester bonds, liberating small, water-soluble monomeric compounds that are rapidly cleared from the body by the kidneys. These compounds do not alter the pH of the local tissue environment.
The novel ER polymer is a component of the FDA-approved SUSTOL (granisetron) ER injection for subcutaneous use and has been extensively evaluated in nonclinical safety studies including repeated-dose toxicity with daily dosing for 90 days, for in vitro and in vivo genotoxicity, and for reproductive and developmental toxicity studies in two species. Additional nonclinical toxicology studies conducted during the development of HTX-011 revealed no findings suggestive of systemic toxicity and no sustained local effects. HTX-011 was also extensively evaluated in local tolerance studies; briefly, HTX-011 did not demonstrate an effect that was meaningfully different compared with bupivacaine HCl on bone healing, skin wound healing, wound healing in the presence of an implant (eg, mesh), or neurotoxicity (data on file).
Initial studies focused on the development of an ER bupivacaine formulation. It is known that the analgesic activity of liposomal bupivacaine diminishes after 24 hours,5 and it was hypothesized that this could be due, in part, to the migration of the liposomes away from the pain-generating area. If that is the case, a polymeric ER bupivacaine formulation administered directly to the wound should remain in contact with pain-generating tissue and provide prolonged analgesia. A polymer-based, 6-day ER formulation, ER-B-6D, produced superior activity but diminishing analgesia after 24 hours; however, an unexpected return of analgesic activity was observed after 96 hours. This return of activity after 96 hours negated the migration hypothesis but was consistent with the expected time course of local inflammation produced by surgical insult. The initial inflammatory response with resulting drop in tissue pH6 and peripheral sensitization subsides with time, with an increasing fraction of bupivacaine in the unionized form available to penetrate the nerve membrane producing a greater analgesic effect.
In theory, local administration of NSAIDs would be expected to limit the local inflammatory response and inhibit the production of tissue prostaglandins, thereby reducing the local inflammatory-mediated tissue pH drop and peripheral sensitization. This could prevent the normal loss of clinical efficacy beyond the 12- to 24-hour window seen with other ER local anesthetics. Screening of various NSAIDs to be coformulated with bupivacaine that may alter local inflammation led to the selection of meloxicam. The combined formulation demonstrated sustained analgesic activity over 144 hours in the pig postoperative pain model, which was not observed with ER bupivacaine alone. In addition, the properties of meloxicam made it ideally suited for inclusion into the formulation: high potency (allowing for low concentrations and better control of drug release), low COX-1 activity with a demonstrated minimal impact on clotting, and low cardiovascular risk, especially given the short duration and low systemic exposure associated with local delivery.24–26 Notably, in the HTX-011 phase III studies in bunionectomy and herniorrhaphy, mean maximum concentrations of meloxicam (Cmax) were much lower compared with the mean maximum concentration of oral meloxicam using the lowest recommended dose in a healthy male population (mean meloxicam Cmax in phase III studies, 31.6 ng/mL and 181 ng/mL [data on file]; Cmax in meloxicam package insert, 1050 ng/mL).27 The results of the assessment of bone healing conducted in the phase II bunionectomy study (data on file) are consistent with studies showing that short-term use of low-dose NSAIDs does not interfere with bone healing.28 29
Demonstration of synergistic activity of bupivacaine and meloxicam
The usual isobologram analysis used to demonstrate synergy requires both ingredients of the combination to have activity on the measured response in order to generate dose-response curves for each individual active ingredient.30 As demonstrated in both the pig postoperative pain model and in the phase II bunionectomy study, the meloxicam component of HTX-011 produced no appreciable analgesic activity, whereas HTX-011 produced significantly greater and prolonged analgesia than either bupivacaine or meloxicam separately. Therefore, the supra-additive analgesic activity of HTX-011 over the ER bupivacaine formulation in the bunionectomy phase II study confirms a synergistic interaction, with meloxicam potentiating the analgesic activity of bupivacaine.
In both preclinical and clinical models, the enhanced analgesic activity of formulations of bupivacaine plus meloxicam over formulations of bupivacaine alone became apparent within the first 5 hours; this was maintained over 72 hours, consistent with the period where inflammation would be impacting the activity of bupivacaine. This is also consistent with the meloxicam component producing a direct effect on the microenvironment of the surgical site, as high systemic concentrations of meloxicam had no impact on the analgesic activity of bupivacaine. This may be due to several factors, such as the need for high local concentrations to reduce inflammation in order to normalize the pH and to limit peripheral sensitization, as well as local ischemia resulting from surgery preventing systemic meloxicam from achieving pharmacologically active concentrations in the tissue. Further studies will be needed to establish the individual contributions of these effects, but the dramatic synergy resulting in significantly increased analgesia is evident in both preclinical and clinical studies.
In summary, HTX-011 is a nonopioid, ER, dual-acting local anesthetic formulation comprising bupivacaine and low-dose meloxicam that uses novel, extended-release polymer technology. Preclinical data demonstrate the ability of HTX-011 to address local tissue inflammation by reducing the drop in tissue pH in the incision after surgery compared with control, which was associated with potentiated and prolonged analgesic activity. The approximately 1 pH unit difference observed at 48 hours results in >10-fold more unionized bupivacaine available to enter the nerve with HTX-011 treatment compared with control. The ability to provide consistent analgesia throughout the critical 72 hours postoperative period may provide an important option for clinicians to manage pain without overreliance on opioids.
Acknowledgments
Assistance with manuscript preparation was provided by Andrew Occiano, PharmD, at ApotheCom (San Francisco, California, USA) and was funded by Heron Therapeutics, Inc. (San Diego, California, USA).
References
Footnotes
Contributors All authors conceived of and designed the study, analyzed and interpreted the data, drafted the manuscript, and provided critical revisions to the manuscript.
Funding Funding for this research was provided by Heron Therapeutics, Inc. (San Diego, California, USA).
Competing interests TO and BQ are employees of Heron Therapeutics and receive salary and stock options. JP received consulting fees from Heron Therapeutics, Acacia Pharma, and Mallinckrodt Pharmaceuticals and is on the speaker’s bureau for Mallinckrodt Pharmaceuticals. JFD receives consulting fees from Heron Therapeutics, AcelRx Pharmaceuticals, Neumentum Pharmaceuticals, Aries Pharmaceuticals, and Pacira Pharmaceuticals. EV receives consulting fees from AcelRx, Concentric, Heron Therapeutics, Innacoll, Merck, Neumentum, Pfizer, Recro, Salix, and Trevena.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available on reasonable request.