Article Text
Statistics from Altmetric.com
Introduction
Burn pain includes not only the burn itself but also the pain associated with procedures such split thickness skin graft (STSG) harvesting.1 Opioid analgesics, conventionally the first-line agent for burn analgesia, are associated with hyperalgesia in burn patients.2 Peripheral nerve blocks may be used for postoperative analgesia following STSG, and the lateral femoral cutaneous nerve (LFCN) is an optimal target, since it innervates the lateral thigh and has no motor component.1 3 Unfortunately, local anesthetic nerve blocks are limited in duration to hours–or days with a continuous infusion.4
Ultrasound-guided percutaneous cryoneurolysis offers an alternative to local anesthetic blocks with a duration of weeks or months.5 A previous case series has suggested that cryoneurolysis of the LFCN may provide extended duration analgesia for STSG donor sites.6 We designed a randomized, controlled pilot study to evaluate the feasibility of percutaneous cryoneurolysis of the LFCN and estimate the treatment effect to plan for a subsequent definitive trial.
Methods
The protocol was approved by the UC San Diego Institutional Review Board (number 180863, San Diego, California) and prospectively registered at clinicaltrials.gov (NCT03578237; Brian M. Ilfeld, MD, MS; 25 June 2018). Enrollment was offered to adult burn patients undergoing STSG with a planned LFCN block. Exclusion criteria included daily opioid use for a duration of at least 4 weeks, morbid obesity, incarceration, pregnancy, and medical contraindications to cryoneurolysis.5
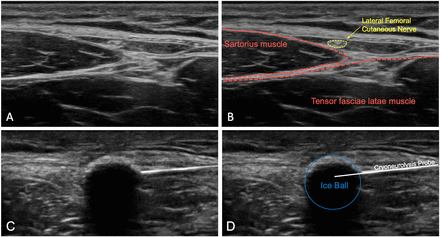
The LFCN was identified in short axis using a linear ultrasound transducer (figure 1A,B) and ropivacaine 0.5% (3–5 mL) was injected circumferentially. Twenty minutes following the block, loss of temperature discrimination to ice was used to map the nerve distribution. If the burn site was an acceptable target for a regional anesthetic, a continuous peripheral nerve block was performed (infraclavicular block for upper extremity; sciatic and/or femoral for lower extremity).
(A) The lateral femoral cutaneous nerve is identified using ultrasound in the intermuscular plane between the sartorius and tensor fasciae latae muscles. (B) The lateral femoral cutaneous nerve (yellow) and sartorius and tensor fasciae latae muscles (red) are labeled. (C) Ultrasound is used to visualize the ice ball completely enveloping the lateral femoral cutaneous nerve, which can no longer be distinguished in the frozen tissue. (D) The sartorius muscle, cryoneurolysis probe, and ice ball are labeled.
After confirming a successful block, patients were randomized to either an Active or Sham cryoneurolysis procedure. While patients were blinded to randomization, the operator could not be blinded as the ice ball was visible on ultrasound in the Active group. A 14G probe connected to a console-based cryoneurolysis machine (PainBlocker, Epimed International, Farmers Branch, TX) was inserted, and the tip was advanced under ultrasound guidance adjacent to the LFCN. The cryoneurolysis machine was activated for three cycles of 2 minutes on and 1 minute off.
Active
The ice ball at the tip of the probe was viewed enveloping the LFCN (figure 1C,D).
Sham
The probe vented the nitrous oxide at its proximal end, thus no freezing occurred at the distal tip.
Intraoperatively, patients received either general anesthesia or sedation if the blocks provided sufficient anesthesia. Postoperatively, patients received scheduled acetaminophen (975 mg every 8 hours) and oxycodone (5–15 mg) as needed with intravenous hydromorphone (0.5 mg) for breakthrough pain.
Worst and average pain at the donor site (Numeric Rating Scale of 0–10) and difficulty sleeping (yes/no) were collected on postoperative days 1–4, 7, 14, and 21 by an investigator not blinded to treatment group. Outcomes at months 1, 3, 6, and 12 were intended but inadvertently overlooked during data collection. Hydromorphone dosing was converted to equianalgesic dosage of oxycodone. As this was a pilot study, a convenience sample of 12 subjects was chosen and only descriptive statistics were applied.
Results
Twelve subjects were enrolled (Active: n=6, mean age 48±17 years, 6 (100%) men; Sham: n=6, mean age 47±13 years, 1 (17%) men). All subjects had a successful ropivacaine LFCN block, underwent surgical STSG, were randomized, and received the study intervention.
Only 9 of 12 subjects could be contacted on postoperative day (POD) 7, 14, and 21. On POD 1–4 and 7, subjects randomized to active cryoneurolysis experienced lower average and maximum pain scores and required less opioid analgesics compared with Sham (table 1). Subjects randomized to active cryoneurolysis were less likely to report sleep disturbances for the first two nights following surgery.
Results
Discussion
This pilot study provides evidence that LFCN cryoneurolysis following STSG is feasible and may result in improved analgesia and lower opioid consumption for 1 week when added to a ropivacaine nerve block. Only marginal differences between groups were noted by the end of the first week and at the 2-week and 3-week time points, likely representing the normal healing process.
The LFCN shares watershed areas with the femoral nerve anteriorly, posterior cutaneous nerve of the thigh posteriorly, and ilioinguinal and iliohypogastric nerves superomedially. Thus, subjects may have experienced pain on the edges of the LFCN distribution. Similarly, the graft sites occasionally strayed from the borders that were delineated or were larger than the LFCN distribution (figure 2).
Graft sites straying outside the block distribution may result in pain not covered by the local anesthetic nerve block or cryoneurolysis.
Cryoneurolysis of the LFCN is feasible and may be a viable option for decreasing pain and opioid consumption in burn patients undergoing STSG. These results suggest that a definitive randomized trial is warranted.
Ethics statements
Patient consent for publication
Footnotes
Contributors All authors contributed to study design, patient recruitment, data analysis, and manuscript preparation.
Funding Epimed International (Farmers Branch, TX) provided the cryoneurolysis devices used for this pilot study.
Competing interests Drs. Finneran, Swisher, and Ilfeld: The University of California has received funding and product for other research projects from cryoneurolysis manufacturer Epimed (Farmers Branch, TX); infusion pump manufacturer InfuTronix (Natick, MA); and a manufacturer of a peripheral nerve stimulation device, SPR Therapeutics (Cleveland, OH). Drs. Godat, Higginson, and Lee; and Mr. Schaar: no additional conflicts.
Provenance and peer review Not commissioned; externally peer reviewed.